Medical Network March 5 News Bladder transitional cell carcinoma (urothelial cancer, transitional epithelial cancer) is the most common urinary tract malignancy and a common type of bladder cancer (about 90% of all cases). Painless hematuria is the most common clinical condition that occurs in 75% of patients with bladder cancer.
Bladder cancer is clinically divided into non-muscle infiltrating tumors (NMIBC) and myometrial infiltrating tumors (MIBC, or metastatic tumors). Most bladder cancers are diagnosed at an early stage, and approximately 78% of patients will relapse within 5 years.
The survival rate of bladder cancer depends on the stage, type and time of diagnosis of the cancer. Metastatic bladder cancer is generally accompanied by a poor prognosis. The 5-year survival rate of patients with stage IV bladder cancer is only 15%.
Whether treatment is appropriate or not determines the level of mortality
High incidence and low mortality in the United States; low relative incidence and high mortality in China
About 1.3 million people worldwide suffer from bladder cancer, with an average of 430,000 new cases each year. In addition, approximately 165,000 people die each year from bladder cancer. Bladder cancer has a high incidence in developed areas, with nearly 60% of patients in developed areas.
Bladder cancer is the 9th most common cancer in the world and the most common malignant tumor of the urinary system. The incidence rate of males is about 3 times that of females , and the incidence rate is the first in urological malignancies.
In 2016, approximately 80,000 patients in the United States were diagnosed with bladder cancer, and the National Cancer Institute (NCI) estimated that 16,000 people died each year. The total number of bladder cancers in the United States exceeds even more in China and India. Due to the proper treatment plan, although the incidence of bladder cancer in the United States is high, the mortality rate is low.
In countries with relatively low morbidity in China, mortality is high due to limited treatment options. According to Chen Wanqing's data on bladder cancer and morbidity and mortality in 32 cancer-registered areas in China from 2003 to 2007, the total incidence of bladder cancer was 6.69/100,000, 7.79/100,000 in urban areas and 2.93/100,000 in rural areas. The city is 2.7 times that of the countryside. The total mortality rate of bladder cancer is 2.53/100,000, which is 2.82/100,000 in urban areas, 1.52/100,000 in rural areas, and 1.9 times that in rural areas.
Traditional treatment options and their limitations
Metastatic and relapsing treatment options are scarce and new treatments are needed
Current standard treatment options for bladder cancer include aggressive surgery for resection, chemotherapy, and radiation. For NMIBC and MIBC, transurethral resection of bladder tumor (TURBT) is the standard therapy, local intravesical instillation chemotherapy and subsequent immunotherapy apply Bacillus BCG (BCG) as adjuvant therapy. For MIBC, cystectomy with or without adjuvant chemotherapy is recommended. In the past few decades, for metastatic cancer, chemotherapy has long been the mainstay of cancer treatment, the most commonly used is related to platinum-containing drugs.
For NMIBC, BCG perfusion is the most effective intravesical instillation therapy, which can effectively reduce recurrence and delay tumor progression. About 80% of bladder cancer is moderate to high risk NMIBC, and the initial manifestation of patients is superficial cancer. However, after resection, the recent tumor recurrence rate is as high as 50% to 90%, and the long-term recurrence rate is close to 100%. Therefore, all NMIBC patients need adjuvant cystic infusion after operation. BCG began to be used in 1980, although the exact mechanism of action of BCG in the treatment of bladder cancer is not well understood, but scientists may have the following two inferences:
One is by releasing inflammatory cytokines such as IL-1, IL-6, IL-12, IL-15, IL-18 and TNF-α, etc., followed by activation and proliferation of various viable cells, including CD8. , T cells, NK, LAK, BAK cells, etc., inhibit the division and growth of tumor cells, this cell activation process is the most complex stage of anti-tumor effect of BCG.
Second, in addition to the above cellular immune pathways, BCG also activates the humoral immune pathway, but because of its short duration, it is generally considered to be in a secondary position.
The research institute conducted a series of sequential treatments for NMIBC patients with chemotherapy and BCG therapy, but the results did not indicate that the combination of drugs was more effective, so there is no guideline recommending the combination of chemotherapy drugs and BCG.
As mentioned earlier, most bladder cancers are diagnosed at an early stage, and approximately 78% of patients will relapse within 5 years. Therefore, the development of new therapies is urgently needed, and the development direction of new treatments for bladder cancer is also mainly concerned with patients who have failed traditional treatment.
The development of new new therapies abroad is in full swing
Monoclonal antibody development is more active, the listed varieties are PD-1/PD-L1
At present, new treatments for bladder cancer include monoclonal antibodies, gene therapy, tumor vaccines, tyrosine kinase inhibitors, and cytokines.
In the new treatment of bladder cancer, monoclonal antibodies have made some progress and are the most active research areas. Five monoclonal antibodies have been approved for the treatment of bladder cancer. The details are shown in Table 1 below.
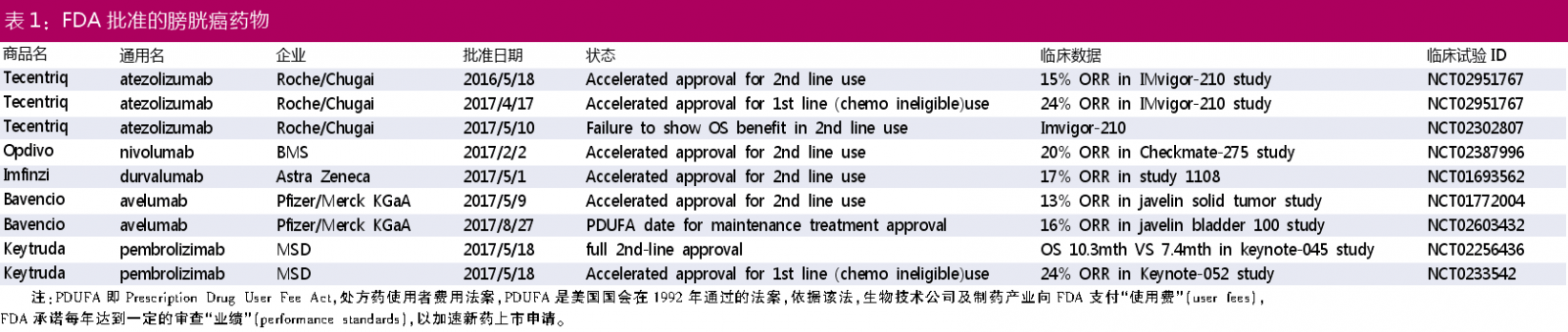
Currently, four PD-1/PD-L1 products that have been approved for marketing by the FDA are: Tecentriq, Opdivo, Imfinzi, and Bavencio.
Among them, Tecentriq (atezolizumab) was accelerated to be approved for first-line and second-line treatment of bladder cancer, but in a follow-up validation study, Tecentriq failed to reach the clinical endpoint of second-line therapy during previous chemotherapy-containing or post-chemotherapy Progression in locally advanced or metastatic urothelial carcinoma (mUC) patients. The data showed that Tecentriq did not reach the primary end point of improving overall survival (OS) compared with chemotherapy, but the safety of Tecentriq was consistent with previous data. Therefore, the future market sales of the product is still unknown.
Merck's Keytruda is the only PD-1/PD-L1 product approved for normal use in bladder cancer. The product is officially approved for second-line treatment of bladder cancer, ie in the treatment of locally advanced or metastatic urothelial cancer, and after platinum-containing chemotherapy or within 12 months of platinum-containing chemotherapy and adjuvant therapy/neoadjuvant therapy Patients whose condition is still progressing. The approval was based on a phase III study in which patients with urothelial carcinoma who had recurrence or progression after platinum-containing chemotherapy were randomized to receive either the PD-1 antibody Keytruda (n=272) or the paclitaxel or doce selected by the investigator. He raced or vinflunine chemotherapy (n=270). The results obtained after 22.5 months of follow-up showed that patients receiving the PD-1 antibody Keytruda group had an overall survival (OS) of approximately 3 months compared with patients receiving the second-line chemotherapy group (median OS was 10.3 months vs 7.4 months). There was no significant difference in median progression-free survival (PFS) (PD-1 antibody Keytruda group 2.1 months vs 3.3 months; HR, 0.96; P=0.32). The researchers said that some patients also benefit from second-line chemotherapy, but these reliefs have a short duration and are generally not toxic for long-term treatment, while Keytruda is well tolerated. In terms of safety, 62.0% of patients in the Keytruda treatment group experienced treatment-related adverse events at all levels, compared with 90.6% in the chemotherapy group.
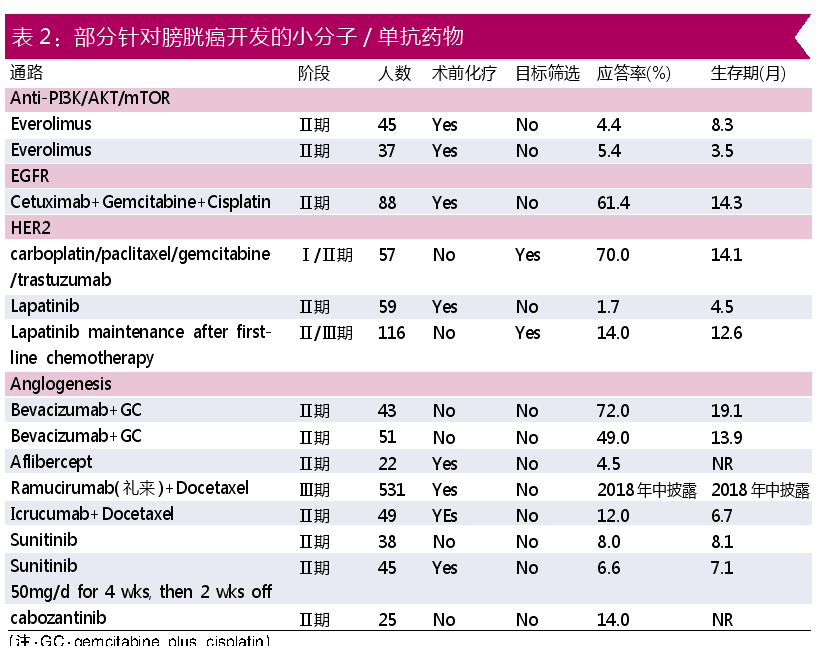
In addition to the PD-1/PD-L1 monoclonal antibody currently available, there are also small molecule drugs, gene therapy, cancer vaccines, and cytokines. In addition to monoclonal antibody therapy, another area of ​​concern for small molecules is the development of small molecule drugs. Table 2 shows some small molecule/monoclonal drugs (produced by Dachang) that have been developed in Europe and the United States.
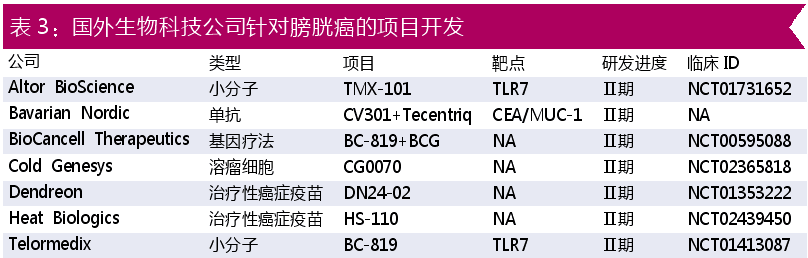
In addition, as shown in Table 3, major foreign biotechnology companies have explored in new therapies such as gene therapy, oncolytic virus, and cancer vaccine. At present, these foreign projects have reached the stage of clinical phase II.
Large domestic research and development imagination
PD-1/PD-L1+ various combination therapy development will become the mainstream of the future
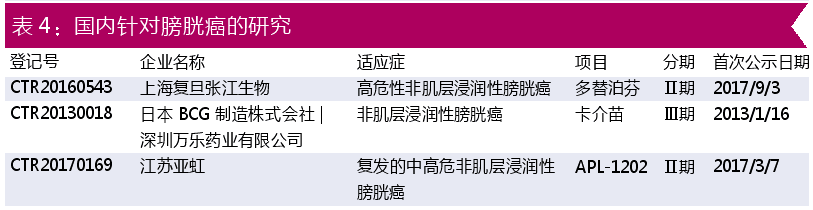
In China, as shown in Table 4, there are three researches on bladder cancer treatment, namely: Shanghai Fudan Zhangjiang Bio, Shenzhen Wanle Pharmaceutical, and Jiangsu Yahong Pharmaceutical . This is only a manufacturer registered in the clinical trial registration platform, and does not include a large number of small-molecule drugs for solid tumors in pre-clinical or clinical phase I projects in China.
Relatively speaking, domestic research on bladder cancer is still relatively small, so there is still a large imagination. The combination of various therapies with PD-1 also gives new treatments more opportunities. For example, Bavarian Nordic's CV301 is developed in collaboration with Roche and in combination with Tecentriq; while Heat Biologics' HS-110 is used in combination with BMS's Opidivo; there are more cases of joint development of marketed drugs and chemotherapy, and BCG. Combination medication. Therefore, combination therapy should be a trend in the future clinical use of bladder cancer. In China, the fastest development of PD-1 is BMP's Opidivo, which has already submitted a listing application; MSD's Ketruda, Roche's Tecentriq and AstraZeneca's Imfinzi project are still in clinical phase III. For bladder cancer, the only one of the currently listed 5 PD-1/PD-L1 has been approved for indications, and may find more possibilities in China.
Conclusion>>>
According to related reports, by 2020, chemotherapy and radiotherapy are still expected to be the most important treatment for bladder cancer treatment, and the market share will reach 76%. Targeted treatment will also gradually emerge, and the market share of this segment will increase year by year.
However, the mortality rate of bladder cancer suggests that traditional therapies (mainly including chemotherapy, radiotherapy, and existing biotherapies) can treat the disease appropriately. However, for metastatic disease, the treatment regimen is still scarce and the patient's quality of life is poor after surgery. For advanced patients with cancer, new therapies may improve clinical outcomes.
However, from the domestic research and development situation, we can see that the current clinical projects are still the original chemotherapy and BCG therapy. The domestic PD-1/PD-L1 project has been approved in China, and there are 2 Junshi Bio and Baekje Shenzhou. The development of bladder cancer is carried out, and it is expected that more PD-1/PD-L1 will be developed in the future after the approval of the bladder cancer.
An insulin pen injector is a medical device used by people with diabetes to inject insulin into their body. It looks like a pen and has a cartridge filled with insulin that is inserted into the device. The pen has a dial that can be turned to select the desired dose of insulin, and a button that is pressed to inject the insulin into the body. Insulin pen injectors are convenient and easy to use, making it easier for people with diabetes to manage their insulin therapy. They are available in both disposable and reusable forms, and can be used with different types of insulin.
Insulin Pen Injector,Insulin Glargine Pen,Disposable Pen,Insulin Pen Cartridges
Shanghai Enjosim Medical Technology Co., Ltd , https://www.enjosimmedical.com