In June of this year, a medical equipment manufacturer in Wenzhou applied for 5 imported medical devices to the Wenzhou Inspection and Quarantine Bureau, which was a cardiopulmonary resuscator with a value of more than 10,000 euros. During the inspection, the staff of Wenzhou Inspection and Quarantine Bureau found that the enterprise could not provide a valid registration certificate for imported medical devices, did not meet the requirements of the Regulations on the Supervision and Administration of Medical Devices, and was found to be unqualified for inspection. The return procedure is in the process of being processed.
Imported medical devices refer to instruments, equipment, appliances, materials or other items that are used in the territory of the People's Republic of China and used in the human body, either alone or in combination, including the software used. Because medical equipment involves safety, health, environmental protection, health and other project requirements, China's relevant laws and regulations have strict quality and safety requirements. The Regulations on the Supervision and Administration of Medical Devices promulgated and implemented by the State Council in 2014 clearly stipulates that imported medical devices should be registered or documented in advance, and should have Chinese manuals and Chinese labels. When applying for registration, enterprises need to submit technical evaluation reports such as product technical evaluation reports and clinical trial data. Unregistered imported medical devices have large risks of quality and safety, and may not be imported according to regulations.
In recent years, as China has increased the public health system and the construction of urban communities and rural primary health care systems year by year, a large number of new and expanded medical institutions have strong demand for equipment. At the same time, the trend of informatization and networking in the medical field has led to an increase in demand for sophisticated medical devices such as intelligence, imaging, and digitization. In terms of high-end medical equipment, there is still a big gap between domestic products and developed countries. At the moment when instrumentation and treatment has become the main means, medical institutions have imported medical devices, especially high-end imported medical devices, as a priority to enhance their strength and enhance their competitiveness, which has greatly stimulated the rapid growth of imported medical devices. At the same time of the rapid growth of imported medical devices, the existing equipment quality problems can not be ignored, mainly: no medical device registration certificate, no Chinese manual and Chinese label, new and old mixed. Some products also have security vulnerabilities in design and technical parameters, resulting in electrical safety, equipment safety hazards and radiation damage. Wenzhou Inspection and Quarantine Bureau will increase the inspection and supervision of imported medical devices, strengthen the inspection and supervision of the whole process of medical device unpacking, installation, commissioning and acceptance to ensure the quality and safety of imported medical devices.
Colored sticky corn is generally white, yellow, red, purple and black, among which white, yellow and purple corn are the basic colors. If the purple gene of the purple-white hybrid "beats" the white gene, it will naturally become purple, and vice versa, so if the two are tied, we will see white and purple corn. Purple can become red and black corn, or as we often say, "red is purple and black is purple".
Among these colorful corn, the most common Yellow Waxy Corn is the most nutritious because it is rich in carotene.
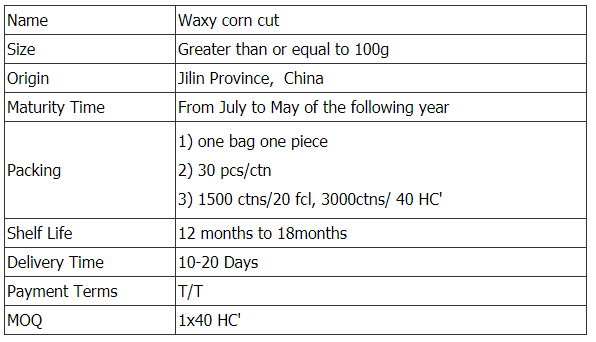


Waxy Corn Cut,Glutinous Corn Cut,Yellow Waxy Corn Cut,Yellow Glutinous Corn Cut
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorn.com