The regulation of multiple life processes in an organism is achieved through the interaction between proteins and proteins. For example, self-assembly of viruses, cell growth, division, differentiation and the like. Usually, the interface of protein-protein interaction is too large, so that it is difficult for small molecule drugs to target them, and the interaction is effectively and specifically blocked, showing good therapeutic effects. Because protein drugs are difficult to pass through the cell membrane, they do not directly target intracellular interactions. Therefore, researchers have begun to seek a solution that can overcome the shortcomings of these two drugs and enter the cell membrane and specific targets. A new drug molecule that interacts with protein-proteins.
Studies have shown that polypeptides with alpha-helical structures and positively charged can cross cell membranes. However, once it is separated from the mother, its original secondary structure cannot be maintained. The instability of the conformation causes its binding to proteins to be weakened, while the ordinary linear polypeptide cannot pass through the cell membrane and is easily hydrolyzed. After repeated attempts, Verdine et al. developed a novel structure of a peptide called a staple peptide, which is an all-carbon scaffold with an α-helical structure and an all-carbon scaffold that stabilizes the α-helical structure. The interaction between the polypeptide molecule and the protein is enhanced, and the peptide can pass through the cell membrane and is not easily hydrolyzed, and has higher pharmacological activity than the previous small molecule drugs and protein drugs.
The synthesis of a peptide is different from the synthesis of a common polypeptide by introducing two unnatural amino acids containing an α-methyl group, an α-alkenyl group, and then an olefin metathesis reaction between two unnatural amino acids. Cyclization constitutes a full carbon scaffold that stabilizes the conformation of the α-helical structure, thereby synthesizing the book peptide.
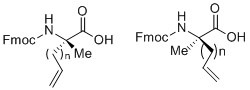
The top panel shows the general structure of an unnatural amino acid containing alpha-methyl, alpha-alkenyl groups in two different configurations. This type of amino acid synthesis is generally:
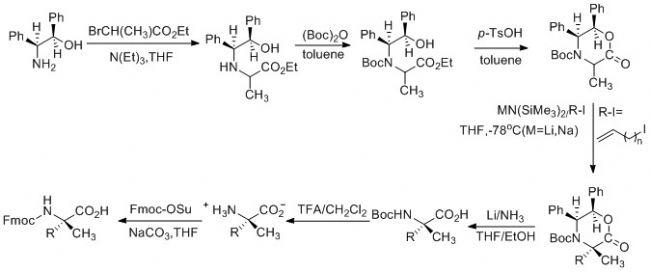 The general synthetic route for a peptide is:
The general synthetic route for a peptide is:
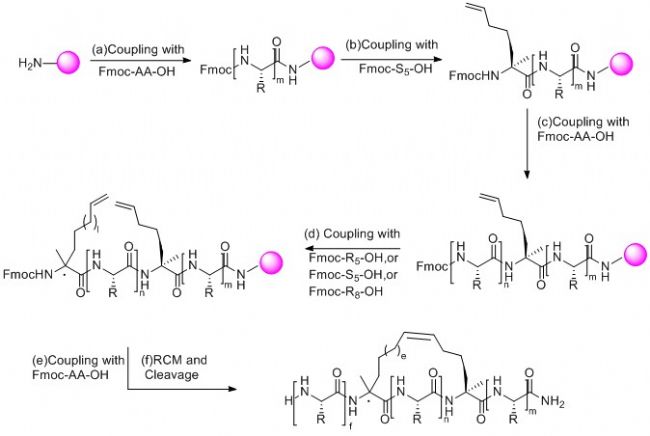 National Peptide Biotechnology always adheres to the customer-oriented business philosophy. Through long-term experimental accumulation, continuous optimization of synthesis conditions and purification processes, it has a mature synthesis process of peptides, and has the ability to provide high-quality peptides to the world. Can fully meet the various research and development needs of customers.
National Peptide Biotechnology always adheres to the customer-oriented business philosophy. Through long-term experimental accumulation, continuous optimization of synthesis conditions and purification processes, it has a mature synthesis process of peptides, and has the ability to provide high-quality peptides to the world. Can fully meet the various research and development needs of customers.
success case:
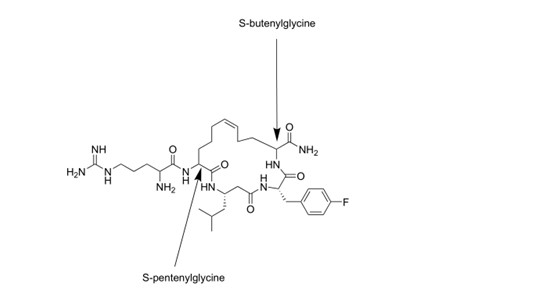
Synthesis of the following structural peptides
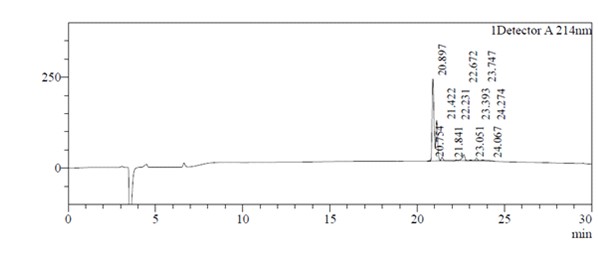
HPLC analysis:
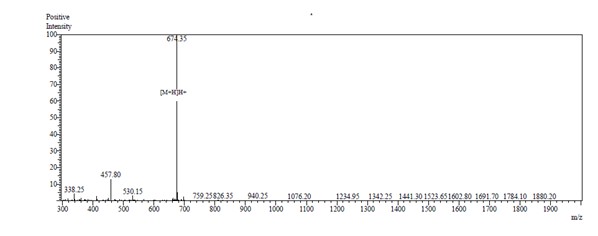
 MS analysis:
MS analysis:
Studies have shown that polypeptides with alpha-helical structures and positively charged can cross cell membranes. However, once it is separated from the mother, its original secondary structure cannot be maintained. The instability of the conformation causes its binding to proteins to be weakened, while the ordinary linear polypeptide cannot pass through the cell membrane and is easily hydrolyzed. After repeated attempts, Verdine et al. developed a novel structure of a peptide called a staple peptide, which is an all-carbon scaffold with an α-helical structure and an all-carbon scaffold that stabilizes the α-helical structure. The interaction between the polypeptide molecule and the protein is enhanced, and the peptide can pass through the cell membrane and is not easily hydrolyzed, and has higher pharmacological activity than the previous small molecule drugs and protein drugs.
The synthesis of a peptide is different from the synthesis of a common polypeptide by introducing two unnatural amino acids containing an α-methyl group, an α-alkenyl group, and then an olefin metathesis reaction between two unnatural amino acids. Cyclization constitutes a full carbon scaffold that stabilizes the conformation of the α-helical structure, thereby synthesizing the book peptide.

The top panel shows the general structure of an unnatural amino acid containing alpha-methyl, alpha-alkenyl groups in two different configurations. This type of amino acid synthesis is generally:
 The general synthetic route for a peptide is:
The general synthetic route for a peptide is:  National Peptide Biotechnology always adheres to the customer-oriented business philosophy. Through long-term experimental accumulation, continuous optimization of synthesis conditions and purification processes, it has a mature synthesis process of peptides, and has the ability to provide high-quality peptides to the world. Can fully meet the various research and development needs of customers.
National Peptide Biotechnology always adheres to the customer-oriented business philosophy. Through long-term experimental accumulation, continuous optimization of synthesis conditions and purification processes, it has a mature synthesis process of peptides, and has the ability to provide high-quality peptides to the world. Can fully meet the various research and development needs of customers. success case:
Synthesis of the following structural peptides

HPLC analysis:
 MS analysis:
MS analysis: 
Extremity Pack,Sterile Extremity Pack,Disposable Extremity Pack,Disposable Sterile Extremity Pack
Xinxiang Huaxi Sanitary Materials Co., Ltd. , https://www.huaximedical.com