February 4th is the 19th World Cancer Day. Faced with the grim situation of cancer prevention and control in China, medical experts have called for increased investment in research and development of new cancer drugs to further improve the accessibility of new cancer drugs in China.
According to the report “Responding to Cancer Challenges by Drug Innovation†recently published by the China Association of Enterprises for Foreign Investment and Investment Industry Committee, the 5-year survival rate of cancer patients in the United States has increased by 41% compared with 1975, and the survival rate of 83% is extended. New treatments including innovative medicines. However, in China, the global cancer drug accessibility rankings in 2010-2014 are far less than those in developed countries such as the United States and the United Kingdom, and only 6 of the 49 new drugs are listed in China.
"For more than 30 years, there have been no effective chemotherapy drugs or targeted drugs to treat esophageal cancer. There is only one type of targeted drug for gastric cancer." Shen Lin, deputy dean of Peking University Cancer Hospital, said at the press conference that due to cancer diseases with Western countries. Different spectrums, esophageal cancer and gastric cancer, which are easily available to Chinese people, are themselves a world problem. The clinical trials of new drugs are prone to failure, resulting in a decline in the willingness to invest in research and development, thus forming a vicious circle.
Due to the rapid development of the health industry, fierce competition has forced many companies to conduct a lot of research simultaneously, so the degree of refinement is insufficient and the probability of R&D failure increases. Shen Lin believes that this is one of the reasons for the slow listing of new cancer drugs. In the past two years, China has begun to take measures to improve the accessibility of new cancer drugs. In 2017, among the 36 kinds of negotiating drugs included in the medical insurance catalogue, 15 western medicines were tumor therapeutic drugs; the State Food and Drug Administration also included the drug registration application for preventing and treating malignant tumors with obvious clinical advantages into the scope of priority review and approval. With the in-depth advancement of medical reform and the accelerated evaluation of innovative drug registration, more and more research and development and listing of innovative drugs for cancer frontiers in China will be synchronized with the international market.
Zhao Yan, vice president of Novartis Oncology (China) Department of Oncology, said that the development of innovative drugs is a systematic project that requires interdisciplinary collaboration between scientists and pharmaceutical companies. Shen Lin believes that increasing the research and development of new cancer drugs and improving the accessibility of new cancer drugs requires the joint efforts of the government, pharmaceutical companies, hospitals and medical staff.
(Source: Xinhua News Agency)
Operating table is used at surgical operation room, our table can use for almost all kinds of surgries; For surgical table, we have three types, electric operation table, electric hydraulic operation table; Manual surgical table; Lewin company do much investment on new products research every year, to research new type surgical table to meet the hospital requirment;we Lewin has High grade surgical table, middle grade surgical table to meet different requirments from user; The OT table adopt imported parts, much stable and safty;

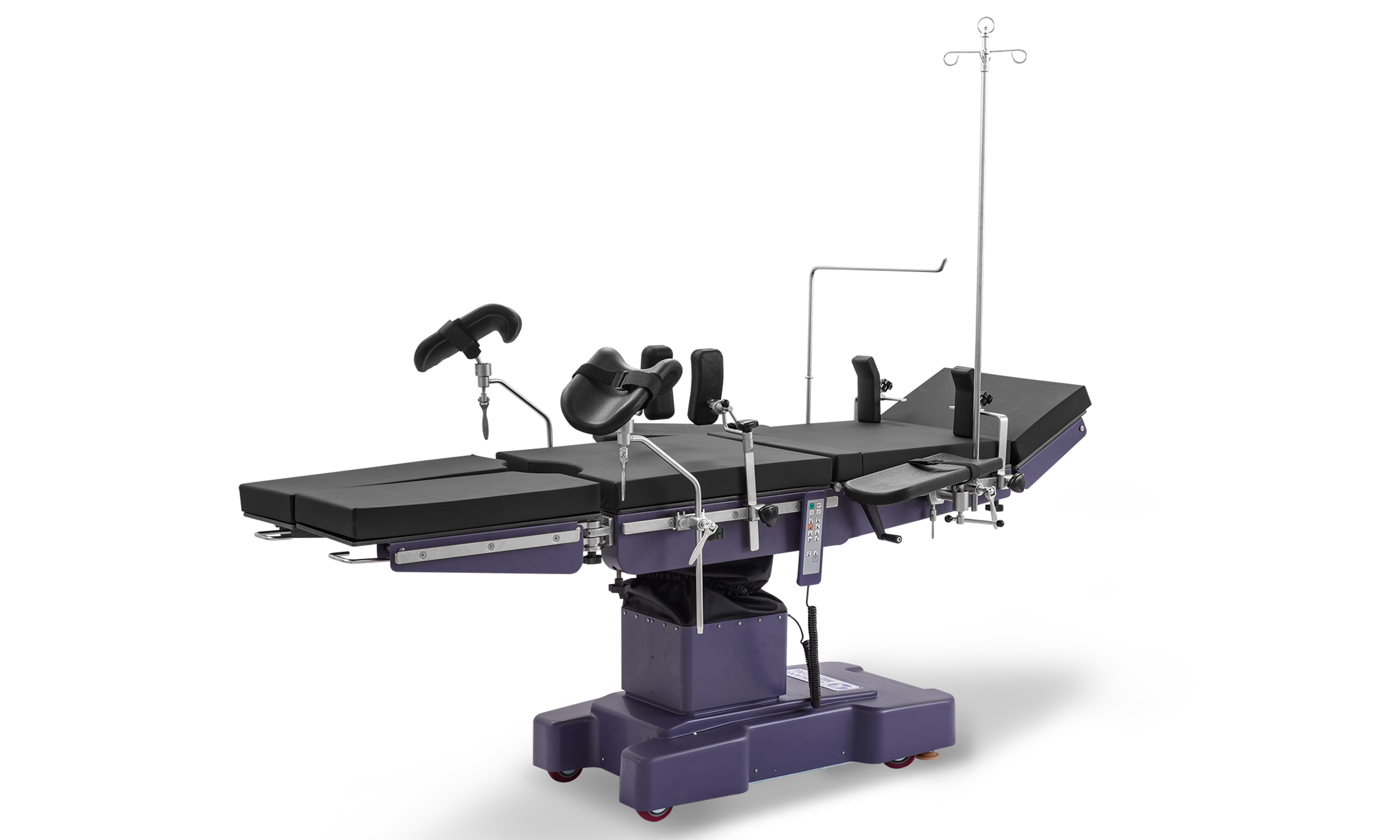

Operating Table,Surgery Table,Surgical Table,Electric Hydraulic Operating Table
Shandong Lewin Medical Equipment Co., Ltd. , https://www.lewinmed.com