Rui Chen, Jinchuan Yang and John McCauley
Waters Corporation (Milford, MA, USA)
Application advantage
Simultaneous separation of nine fat-soluble vitamins and carotene avoids the use of multiple HPLC methods and reduces the number of experimental steps required for qualitative and quantitative analysis of fat-soluble vitamins in dietary supplements and fortified foods. Rapid separation of hydrophobic vitamins and carotene can significantly increase sample throughput and reduce lab costs, thus meeting the increasing regulatory compliance requirements to monitor large amounts of analytical work.
Waters offers solutions
ACQUITY UPC 2 TM System with Photodiode Array (PDA) Detector
Empower ® 3 software
ACQUITY UPLC ® HSS C 18 Column
Key words
Vitamins, carotene, fat soluble, vitamin A acetate, vitamin A palmitate, vitamin D2, alpha-tocopherol, vitamin E acetate, vitamin K1, vitamin K2, lycopene, beta-carotene, Chromatography, UPC 2
introduction
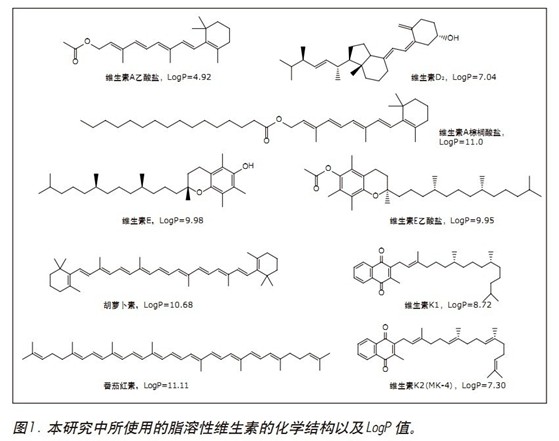
Fat-soluble vitamins (FSV) include vitamins A, D, E, K and carotenoids (eg, beta-carotene). Fat-soluble vitamins are involved in many complex metabolic reactions associated with important physiological functions, such as vision (vitamin A), calcium absorption (vitamin D), cell membrane antioxidants (vitamin E), and blood clotting (vitamin K). 1 Beta-carotene is a precursor of vitamin A and has 100% vitamin A activity in humans. Lycopene is not an essential nutrient for the body, but its antioxidant properties make it popular and are increasingly being added to certain dietary supplements along with other ingredients. The chemical structure of several fat-soluble vitamins is shown in Figure 1.
Vitamins and nutritional supplements are a market worth billions. It is expected that this market will continue to grow in the next five to ten years. 2 This is because consumers around the world are increasingly pursuing better and healthier lifestyles, as well as increasing emphasis on health and eating habits. However, people are also paying more and more attention to the safety of fat-soluble vitamins taken from nutritional supplements and nutritionally fortified foods, especially vitamins A and D, which, if consumed in excess, pose serious health risks. 3 Since many laws on the regulation of trace elements added to nutrient fortified foods and dietary supplements are being developed or formulated in many countries, it is inevitable that the market will be able to quickly and accurately analyze the fat solubility in different products. There is a higher demand for analytical methods for vitamin content.
At present, in the FSV separation, the most commonly used liquid chromatography (LC) method, both reverse (RP) and forward (NP) methods. 1,3-5 Although the AOAC method can be used for qualitative and quantitative analysis of various fat-soluble vitamins in foods and nutritional supplements, but lacks a kind of fat-soluble vitamins and carrots in vitamin premixes. The method of simultaneous analysis. 3
Because ultra-high performance convergent chromatography (UPC 2 TM) is fast, economical and durable, it can be considered for rapid analysis of pharmaceutical preparations containing various fat-soluble vitamins. 6 In this application note, we describe a single injection method that simultaneously separates nine fat-soluble vitamins in four minutes. The optimized method has good reproducibility in terms of retention time and peak area and can be used for high throughput quantitative analysis.
experiment
sample discription
Seven fat-soluble vitamins: retinol acetate (vitamin A acetate), retinyl palmitate (vitamin A palmitate), alpha-tocopherol (vitamin E), alpha-tocopherol acetate (Vitamin E acetate), ergocalciferol (vitamin D2), vitamin K1, vitamin K2 (MK-4); and two carotenoids: lycopene and beta-carotene were purchased from Sigma Aldrich ) and used directly without processing. All samples were dissolved in methyl tert-butyl ether (MTBE) at approximately 0.1 mg/mL and transferred to a sample vial for analysis.
UPC 2 conditions
System: ACQUITY UPC 2 with PDA detector
Flow rate: 1 mL/min
Mobile phase A: CO 2
Mobile phase B: acetonitrile
Column: ACQUITY UPLC HSS C 18 3.0 x 100 mm, 1.8 μm
Back pressure: 2500 psi
Cylinity: 30 °C
Sample diluent: MTBE
Injection volume: 1μL
Vial: Waters Glass 12 x 32 mm Threaded Bottle, 2 mL
PDA scanning range: 210 - 600 nm
Data Management: Empower 3 Software
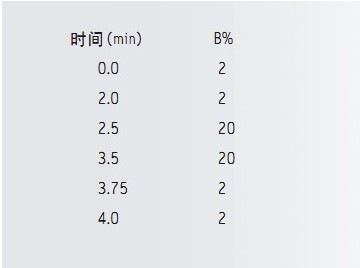
gradient:
Results and Discussion <br> A large number of literatures have been used to describe the separation of fat-soluble vitamins and carotenoids using NPLC and RPLC. 1, 3-5 Since the analysis of fat-soluble vitamins usually requires the use of low-polarity organic solvents to dissolve the sample, NPLC has certain advantages due to its compatibility with organic solvents, allowing direct injection of fat-soluble vitamins or A fat-soluble vitamin extract without the need for an evaporation step. Although the chromatographic separation efficiency of the RPLC method is higher than that of NPLC, 7 it hinders its application in FSV analysis for the following reasons: 1) the analyte has low solubility in the mobile phase, and 2) the retention of FSV is strong. , causing the running time to be too long. Finally, one chooses to use a semi-aqueous mobile phase (methanol or a mixture of acetonitrile and water) or a non-aqueous mobile phase; the latter is also known as non-aqueous reversed phase (NARP) LC. 7-8 UPC 2 , although often used as a normal phase separation technique, is equally applicable to the separation of analytes of lower polarity. The main mobile phase CO2 of UPC 2 is not only a good solvent for low-polarity analytes (similar to the polarity of hexane), but also an organic solvent used to dissolve these analytes during sample separation (eg in this study) The MTBE used is the same. In addition, the low polarity of CO 2 also promotes non-polar interactions between the analyte and the mobile phase, thereby reducing retention time and run time.
In the development of the method, six columns were selected: ACQUITY UPLC HSS T3, ACQUITY UPLC HSS C 18 , ACQUITY UPC 2 HSS SB, ACQUITY UPC 2 CSH fluorophenyl, ACQUITY UPC 2 2-ethylpyridine, and ACQUITY UPC 2 BEH,
And four solvents, including: isopropyl alcohol (IPA), acetonitrile (ACN), ethanol, and a 1:1 mixture of IPA / ACN. Very similar vitamins K1 and K2 can be separated only by using the ACQUITY UPLC HSS C 18 column and ACN. The lower column temperature, 30 °C, is used for the following reasons: first, to increase the selectivity of K1 and K2; secondly, to minimize the degradation of carotene on the column, because carotene is very susceptible to oxidation.
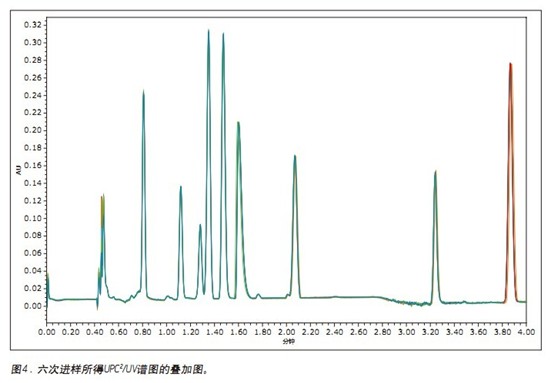
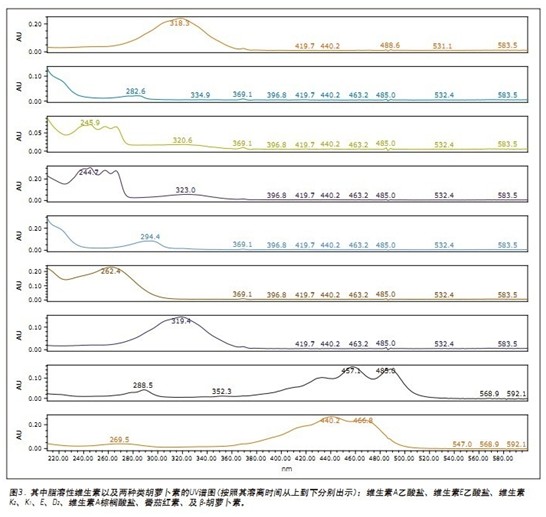
The chromatogram and UV spectrum of each component obtained by the optimization of the method are shown in Figures 2 and 3, respectively. In general, the elution order of the nine fat-soluble vitamins is ordered in the same order as their LogP values. The seven vitamins are all eluted using a low acetonitrile content (2%) of the mobile phase, while the two carotenoids require a mobile phase containing 20% ​​acetonitrile to elute. This phenomenon can be explained by the structure of the analytes and their interaction with the mobile/stationary phase. In all nine analytes, there is a non-polar interaction between CO 2 and the lipophilic vitamin lipophilic group, while the oxygen atoms (in the form of a carbonyl or carboxyl group) of the seven vitamins allow the analyte to interact with the mobile phase. A polar interaction occurs between the acetonitriles in the process, which reduces the dissolution time. The two carotenoid molecules do not contain heteroatoms, and the non-polar octadecyl carbon chain in the molecule is more likely to form a stronger interaction with the stationary phase, and thus the dissolution time is longer.
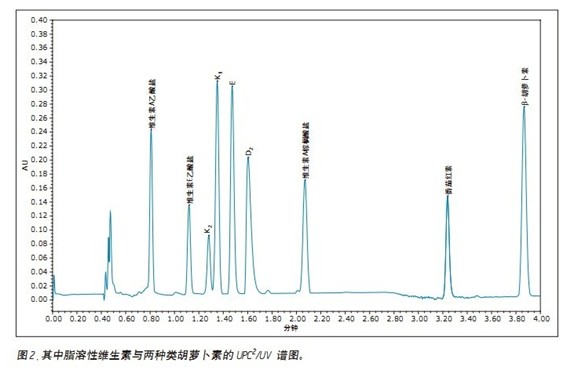
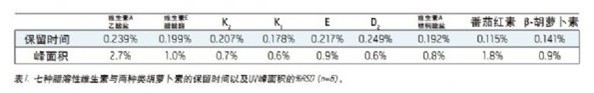
To test the reproducibility of the method, nine fat-soluble vitamins were injected six times, as shown in Figure 4. Reproducibility statistics are summarized in Table 1. The relative standard deviations (RSDs) of retention times are less than 0.25%. In terms of peak area, seven of the nine fat-soluble vitamins have less than 1% RSDs. The RSD of the first peak (vitamin A acetate) is slightly larger because the baseline increases as the number of injections increases. In any case, reproducibility should meet the requirements of quality monitoring, which typically requires a reproducibility tolerance of ±20% for a test method. 3
in conclusion
In this application note, we describe a method for simultaneously separating nine fat-soluble vitamins in four minutes using a single injection UPC 2 method. After six repeated injections, the RSD of the retention times of the nine substances was less than 0.25%, and the RSD of the peak area was less than 3% (<1% in most cases). Using the UPC 2 method, the analysis of the various fat-soluble vitamins in the mixture can be done in one injection without the need for multiple operations like the LC method, which greatly reduces the workload in the laboratory. The time required for the UPC 2 method is between one quarter and one tenth of the traditional analytical method. Because the UPC 2 analysis method is faster, it is suitable for regulatory compliance monitoring, such as food companies/nutrition supplement companies.
references
1. Santos J, Mendiola JA, Oliveira MBPP, Ibanez E, Herrero M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A. 2012; 1261: 179-188.
2. http://
3. Blake CJ. Status of Methodology for the Determination of Fat-Soluble Vitamins
In Foods, Dietary Supplements, and Vitamin Premixes, J. AOAC Inter. 2007; 90(4): 897-910.
4. Gomis DB, Fernandez MP, Gutierrez Alvarez MD. Simultaneous determination of fat-soluble vitamins and provitamins in milk by microcolumn liquid chromatography, J. Chromatogr. A. 2000; 891:109-114.
5. Salo-Vaananen P, Ollilainen V, Mattila P, Lehikoinen K, Salmela-Molsa E, Piironen V. Simultaneous HPLC analysis of fat-soluble vitamins in selected animal products after small-scale extraction, Food Chem. 2000;71:535 -543.
6. Aubin A. Analysis of fat-soluble vitamin capsules using UltraPerformance Convergence Chromatography. Waters Application Note 720004394en. 2012 June.
7. Nelis HJCF, De Roose J, Vandenbaviere J, De Leenheer AP. Non-aqueous reversed-phase liquid chromatography and fluorimetry compared to determination of retinol in serum. Clin. Chem. 1983; 29(7): 1431-1434.
8. Parris NA. Non-aqueous reversed phase liquid chromatography: a neglected approach to the analysis of low-polarity samples. J Chromatogr. 1978; 157:161-170.
Freeze-dried Half Of Fruit
Freeze-drying powder process includes: fresh fruit selection - cleaning - cutting - blanching - cooling - drying - grinding beating - gel grinding - sterilization - pre-freezing - vacuum freeze-drying - ultra-fine grinding - weighing packaging - testing - finished product.
Firstly, fresh and mature fruits with good quality were selected in the pre-treatment area of the food lyophilizer, and the leaves were removed to remove impurities. Then, preliminary cleaning and simultaneous sterilization of fruit surface were carried out. After sterilization, the fruits were cleaned and drained again. After the drain, the fruit is sliced and treated by blanching process. After the color preservation, the fruit is treated by pulping, and then pasteurized. The pretreatment process is finished before lyophilization.
The processing core of freeze-drying powder is the equipment area of food freeze-drying machine. After pre-treatment, the fruit enters the equipment of food freeze-drying machine and crystallizes into a solid state at low temperature, and then sublimates and dries in a vacuum environment to obtain freeze-drying products with little water content. Freeze-dried products out of the warehouse into the food freeze-dried post-processing area, ultrafine grinding, and then according to the need to do weighing packaging, after testing the finished products packed into storage.

Freeze-Dried Half Of Fruit,Mulberry Fruit Powder,Freeze Orange Powder,Raspberry Freeze Dried Powder
Shaanxi HuiKe Botanical Development Co.,Ltd , https://www.oasis-hk.com