It is said that this western blot solution is very good.
Western blot is a commonly used protein analysis method in biochemistry and immunogenetics. It has many technical links, long operation time, and which problems occur, which will affect the final result. Today, Xiao Bian summarizes the WB blotting membrane and gel. The problems and solutions that arise in the hope that can help the little friends who are troubled in WB.
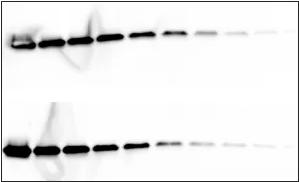
Cause: uneven transfer
Solution: When assembling the sandwich structure, make sure that the parts of the transfer stack are tightly joined together. It may be necessary to replace the sponge pad to achieve the proper tightness.
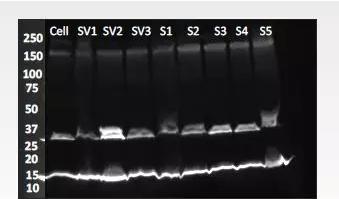
Causes:
1. There may be cell debris or DNA in the sample.
2. The loading buffer is not fresh or contains too much salt.
3. Run the gel too fast, causing the gel to heat up and degrade the polymer.
Solution:
1. Add ultrasound steps
2. Extend heating time
Centrifugation
4. Try to prepare fresh buffer, use different types of buffer, or reduce the salt concentration of the sample buffer by dilution, dialysis/microdialysis or chromatography.
6. Select the appropriate buffer
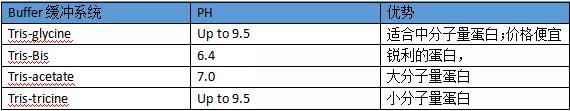 7. Set the appropriate current and keep the voltage low
7. Set the appropriate current and keep the voltage low 
Problem: There are multiple protein bands in the lane
Causes:
1. Incomplete closure
2. Low specificity of primary antibody
3. The concentration of primary antibody is too high
Solution:
1. Replace the blocking solution. Many commonly used blocking fluids can mask the epitope on the target, making it difficult to detect. Blocking is incomplete and non-specifically binds to unrelated lytic proteins. Replacing milk with a commercial blocking agent can reduce non-specific binding while enhancing the interaction of the antibody with the antigen.
2. Purify the primary antibody or replace the antibody
3. Increase the dilution ratio or incubation time of the primary antibody, 4 degree incubation can reduce non-specific binding
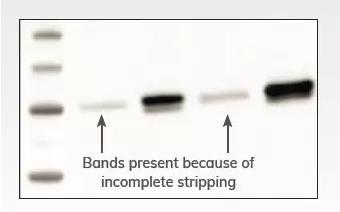
Cause: the peeling is not complete
Solution:
1. Optimize the stripping conditions and increase the stripping time. It is necessary to ensure that the blotting membrane is completely submerged in the stripping buffer and shaken fully on the shaker.
2. Another solution to consider is to perform multiple Western blots using fluorescently labeled secondary antibodies. With fluorescence detection, you can detect up to four different signals, depending on your detection system.

Causes:
1. There are bubbles between the membrane and the gel.
2. The primary antibody is not in contact with the blot.
Solution:
1. When performing sandwich assembly, care should be taken to completely remove air between the blotting membrane and the gel. Gently squeeze the air sandwiched between the blot film membrane and the gel using a glass rod or plastic pipette.
2. Increase the volume of the primary antibody incubation solution and verify that the solution completely covers the membrane during the incubation step. The incubation plate should be large enough to allow the blotting membrane to move.
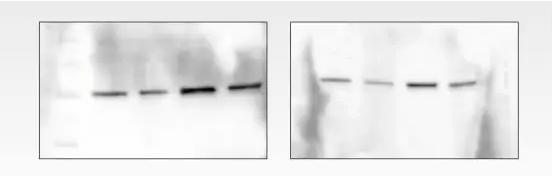
Question: high background
Causes:
1. Insufficient closure
2. Insufficient rinsing
2. The concentration of primary antibody is too high
Solution:
1. Replace the blocking solution. Many commonly used blocking fluids can mask the epitope on the target, making it difficult to detect. Blocking is incomplete and non-specifically binds to unrelated lytic proteins. Replacing milk with a commercial blocking agent can reduce non-specific binding while enhancing the interaction of the antibody with the antigen.
2. Increase the number of rinses or wash time. In addition, the background can be further reduced by increasing the amount of rinse agent or by using a stronger rinse such as SDS or NP-40.
3. Increasing the dilution of the primary antibody or increasing the incubation time, if necessary, incubation at 4 °C can reduce the binding of non-specific antibodies.
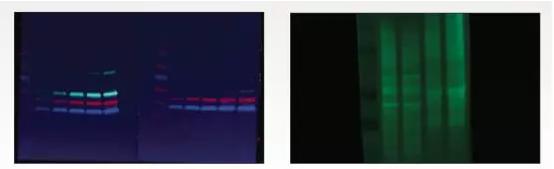
Problem: High background of fluorescent western blot
Causes:
1. NC films and certain types of PVDF films have autofluorescence, resulting in high background signals.
2. Wet film imaging
3. High concentration of primary antibody leads to high background
4. Insufficient rinsing
Solution:
1. Use low fluorescent background film
2. Dry the membrane with methanol before imaging. When the blotting membrane is dry, the autofluorescence on the membrane is reduced and the specific signal emitted by the fluorophore is brightened.
3. Increase the dilution of the primary antibody or increase the incubation time. If necessary, incubate at 4 °C to reduce the binding of non-specific antibodies.
4. Increase the number of rinses or wash time. In addition, the background can be further reduced by increasing the amount of rinse agent or by using a stronger rinse such as SDS or NP-40.
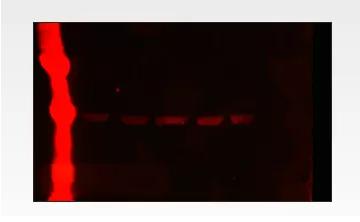
Cause: Marker has too much sample loading
Solution:
1. Increase the dilution of Marker. Most Markers have autofluorescence in the IR 700 channel, which facilitates the determination of the molecular weight of the bands in fluorescent western blots. However, if you are used to using chemiluminescent Western blots, your Marker may be loading too much - we recommend using about one-tenth the molecular weight marker on fluorescent Western blots.
2. We recommend covering strong strips with non-fluorescent, clean, opaque materials.
Dried Fruits,Dried Wolfberry,Dried Strawberry,Dried Lemon
Jiangsu Tiankang Food Co., Ltd. , https://www.tiankangfood.com