In recent years, it seems that every year is a big year of medical policy. Every year, policies that have far-reaching impact on the development of the industry are issued. The competent authorities issue articles and issue texts everywhere, and the interpretations follow suit, and the impact continues to be fulfilled.
According to different regulatory bodies, we divide the policy into three categories: medicine, medical services, and medical insurance. The following is the article catalog:
Medical policy: innovative drugs are imperative, and the value of generic drugs is returning;
Medical services: the promotion of comprehensive reforms in public hospitals, and the promotion of private medical care;
Medical insurance policy: open source and reduce expenditure, the reform of payment methods is carried out in the end.
Innovative medicine is imperative, and the value of generic drugs returns
In recent years, there are two main lines of medical policy, one is to encourage innovation: involving reform of the examination and approval system, the system of listing license holders, CRO, CMO, and the integration of innovative drugs into medical insurance negotiations, from innovative drug research and development, market access to payment. Encourage; the other main line is the integration of stocks, such as consistency evaluation, new version of GMP, two-vote system, drug ratio control, limit resistance and auxiliary, etc., mainly related to generic drug companies, the value of generic drugs returns.

Innovative drug encouragement policies are not limited to domestic enterprises, and foreign companies are treated equally, and multinational pharmaceutical companies can also enjoy the dividends brought by the priority review and approval. First, the import speed of new foreign drugs is accelerating. For example, on July 10, 2018, the State Food and Drug Administration issued the Technical Guidelines for Accepting Clinical Clinical Trial Data of Drugs, and it is conditionally accepted for overseas clinical trial data.
On August 8, 2018, 48 clinically urgently needed new drugs, those that have not yet been declared or are in clinical trials in China, may submit or supplement all research materials obtained abroad if the applicants believe that there is no ethnic difference. There is no supporting material for ethnic differences, and the application for listing is directly submitted. CFDA will speed up the review and approval according to the priority review and approval procedures.
Under the imported innovative drug “Green Passâ€, the number of imported new drugs has increased significantly. According to the GF Securities Research Report, since 2000, the time interval between the approval of new drugs approved by the FDA and the approval of NMPA has been significantly shortened. Since 2012, a total of 91 imported new drugs have been approved for entry into the domestic market; among them, imported new drugs that have been approved for entry into the domestic market since 2017 have increased.
Imported new drugs approved by NMPA since 2012
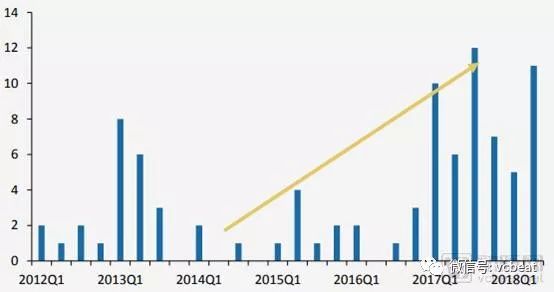
Source: NMPA, GF Securities Development Research Center
In addition, medical insurance strengthens the coverage of innovative drugs. Since 2016, three rounds of national medical insurance negotiations have been completed: in May 2016, three drugs were selected; in July 2017, the Ministry of Human Resources and Social Sciences included 36 drugs in the national basics. Category B of medical insurance, industrial injury insurance and maternity insurance drug list; in October 2018, the National Medical Insurance Bureau included 17 kinds of anticancer drugs in the national basic medical insurance, industrial injury insurance and maternity insurance drug list. Accelerate the entry of therapeutic drugs such as innovative drugs into medical insurance, and in the future may establish innovative drugs into the dynamic adjustment mechanism of medical insurance.
In October 2018, the new anti-cancer drugs that were included in the medical insurance were many newly listed drugs in the past two years, and the time interval between the initial import of many imported varieties was greatly shortened, and they were included in the medical insurance catalogue through price negotiations. The earth has improved the accessibility of domestic patient groups to international first-line drugs for related diseases; in addition, the medical insurance payment price formed by this medical insurance negotiation has generally fallen by more than 50% compared with the previous market price, and some varieties even reached 80%. Decline.
Domestic enterprises are also actively following up the layout of innovative drugs. Capital assistance is an important performance. According to the database of arterial networks and public data, more than 300 domestic new drug companies have been financing since 2014, involving an amount of about 32 billion yuan, and the average amount of financing alone. Nearly 100 million yuan. The rising star of innovative medicines such as Baekje Shenzhou, Cinda Bio, Junshi Bio, and Gloria Pharmaceuticals has emerged.
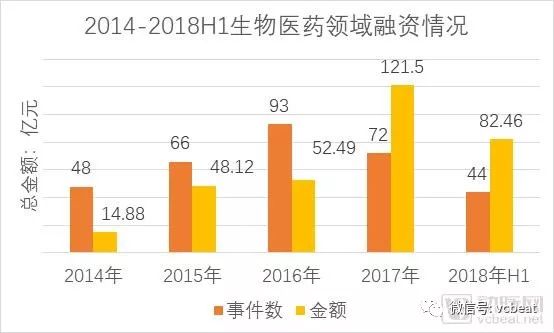
Innovative corporate financing statistics in the field of pharmaceutical and biological fields (partial)
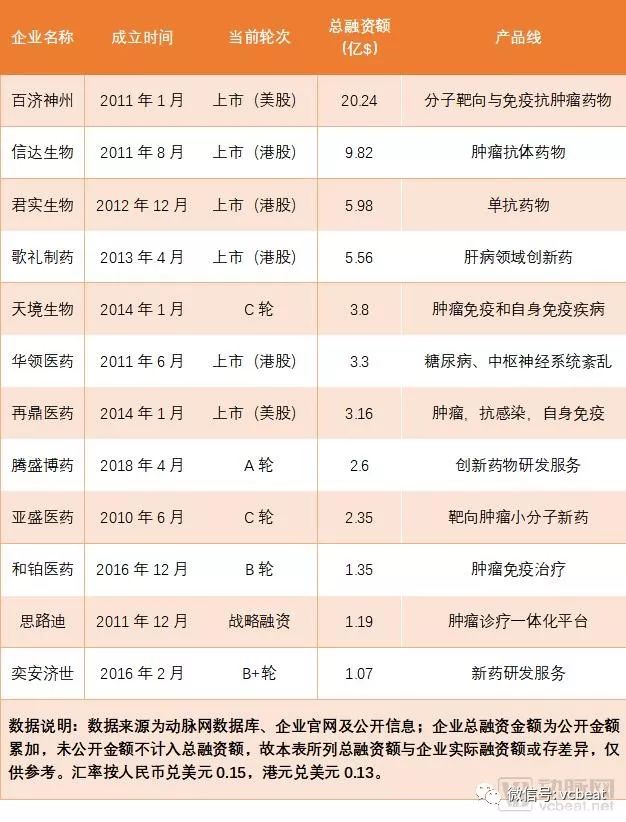
Innovative medicines and old brands Hengrui Medicine, Shijiazhuang Group and Kanghong Pharmaceutical have already matured the path of innovative medicine development. By continuously increasing investment in research and development pipelines, improving research and development efficiency, obtaining high-quality pipelines under development, and actively expanding overseas markets. Or License In/out to help with its innovative drug layout.
Despite the impetuousness of innovative drugs, drug development has the characteristics of long cycle and large investment. At present, there are fewer approved products. Taking PD-1 products as an example, only Junshi and Cinda were approved. Therefore, the “fundamental†of generic drugs in the domestic pharmaceutical market is still stable. According to IMS data, brand generic drugs accounted for 20% in the hospital market in 2017, and ordinary generic drugs accounted for 58%, respectively, at 181.4 billion yuan and 526 billion yuan. The total accounted for about 78% of the hospital drug market.
Comparison of research and development strength of pharmaceutical companies
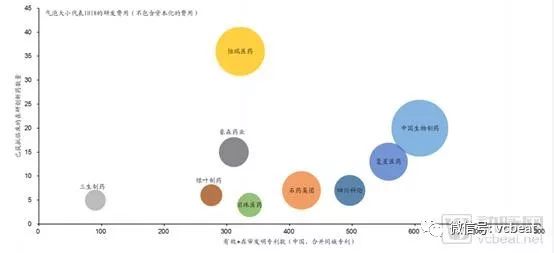
Source: PDB, company information, GF Securities Development Research Center
At present, the biggest challenge facing generic drugs comes from the volume procurement. On December 6, 2018, the results of the bidding for the centralized procurement of 4+7 cities were announced, and the 31 varieties with the quantity purchased finally won 25 bids and 6 discarded. From the price of winning the bid, the decline exceeded the previous market expectations (price reduction of 30%-40%). Among them, there are 10 with a price cut of more than 70%, 8 with a price cut of 40%-70%, and only 7 with a price cut of less than 40%.
Cosmetics Products,Cosmeticghk-Cu Raw Powder,Copper Peptide,Ghk-Cu Powder Cosmetics
Shaanxi Hongbaiyi Biotech Co., Ltd. , https://www.sxhongbaiyi.com