The birth of CAR-T therapy has brought new hope to cancer patients in the name of miracles, which in turn has brought another investment fever. This is also an area where domestic power can be overtaken by corners. If the first two years of clinical and experimental safety and effectiveness exploration is the 1.0 era of China's CAR-T, then the regulation and approval of CAR-T as a drug means the beginning of China's CAR-T 2.0 era. .
In the era of CAR-T2.0, domestic enterprises want to bend over the road, realize the industrialization of CRA-T therapy, how to bypass intellectual property barriers, and break through the common technology of cell pharmaceutical industrialization such as large-scale production process of plasmids and viruses. Point, the establishment of product core competitiveness has become a new challenge, domestic companies to take advantage of the latecomer, to provide CAR-T therapy overall solution to become a new match point.
Among the many suppliers of CAR-T therapy, Shenzhen Purikin is the only one who integrates the complete nano-antibody platform. Prejin has two brands of nano-antibody research and development platform and CAR-T research and development platform. The nano-antibody platform can not only add CAR-T therapy, but also use its advantages to develop more perfect products, and can also form medicines independently to form a nano-antibody industrial chain.
Focusing on the underlying technology of CAR-T drug distribution, taking the road of independent intellectual property rights
The apparent clinical advantages of CAR-T therapy are driving biopharmaceutical companies and investment institutions to compete for their commercialization. Data from Southwest Securities predict that the global CAR-T cell treatment market for hematoma may be nearly $30 billion, or the Chinese market may reach 10 billion yuan. At present, CAR-T therapy has achieved good results in the treatment of hematoma. Once it has made breakthroughs in the treatment of solid tumors, it is expected to capture the global market of billions of dollars of solid tumors.
With the successive introduction and continuous improvement of the CFDA policy regulation in 2017, the path of cell therapy and commercialization has become increasingly clear, and it has become a powerful boost for the development of the industry.
Although there are all kinds of benefits, the success of CAR-T therapy only cures a kind of ending, and there are countless kinds of failures from the laboratory to the landing. Whether it is the barrier of intellectual property blockade or the threshold of production technology, the industrialization process of CAR-T therapy can be accidentally aborted and rejected from the patient.
In order to break through various barriers, one of the main domestic business models is the introduction of foreign products and process systems. Although this business model can temporarily solve intellectual property problems, introduce processes, ensure the maturity of all aspects of products, technology, and quality control, so that CAR-T therapy, which is easy to die in the market promotion process, can be safely industrialized. However, the introduction of foreign patents will reduce the bargaining space of products to a certain extent, and enterprises are relatively lacking in the ability to build underlying technology and product innovation.
And Prygin has taken a path of less choice. Purikin deeply cultivates the underlying technology layout of CAR-T and Nano-antibody, and is committed to the research and development of the source. It uses the industrialization platform to complete the transformation from scientific research to the development of new drugs, and deeply understands the essence of “drugsâ€. Mastering independent intellectual property rights, Prehkin can reduce the cost of preparation processes and master the ability to provide CDMO services to other CAR-T companies. After sturdy in the underlying technology of CAR-T medicine, Prejin also has an advantage in the research and development of the precipitation and the rich product pipeline.
Break through the key nodes of industrialization and provide CAR-T overall solution
Focusing on CAR-T as a drug, Prejin has carried out in-depth and long-term layout and research on the core links of intellectual property, technology, quality, safety assessment and CGMP system that must be solved, and gradually break through the key nodes of industrialization technology to form intellectual property rights. CAR-T overall solution.
Puruijin invested in the establishment of alpaca breeding and antibody immunization research and development base, built a completely independent nano-antibody technology platform, and independently mastered the entire line of antigen preparation, immune antigen, database construction, antibody production of nano-antibody industrialization technology, the production cost Reduced to 1/10 of the average antibody, reaching the quality control standard.
From the perspective of intellectual property rights, Purikin's carrier is designed for independent innovation. Antibodies are also autonomous Nanobodies. In order to obtain fully independent proprietary antibodies, the company invested in the establishment of an alpaca breeding and antibody immunization research and development base, and the animals purchased can be traced.
The CAR-T process has three links, including plasmid, lentivirus and CAR-T cell preparation, including yield and quality. Prehkin has achieved real industrialization earlier. Preqin currently uses a fermentation process to achieve a yield of 100 mg/L after extraction and purification. The use of suspended serum-free packaging technology for lentivirus production greatly increases the yield and is easier to operate, with a yield of hundreds of people. In the preparation of CAR-T cells, Puruijin improved in cell expansion and culture to the blocking process, and independently developed a variety of cell cryopreservation preparations. Dozens of quality standards for plasmids, viruses, and CAR-T cells are set and tested according to CDE documentation requirements.
Moreover, Prejin's CAR-T preparation process is relatively inexpensive, and 50-100 ml of peripheral venous blood is used to prepare CAR-T treatment-related cancer.
In the aspect of safety appraisal, under the witness of the National Shanghai New Drug Safety Evaluation Research Center, Purikin signed a strategic cooperation agreement with Shanghai Enox Biotechnology Co., Ltd. to jointly conduct non-clinical safety evaluation research of CAR-T series products, which guaranteed The authority of the company's product safety assessment.
In the construction of CGMP system, the company has carried out systematic transformation of the existing 2,000-square-meter purification workshop, and recruited and trained dozens of quality and system experts to conduct standardized management to comply with the GLP specifications.
As the first echelon of domestic CAR-T cell drug research and development, Prygin is clearly positioned as the world's leading CAR-T cell drug professional company that integrates nano-antibody platform. At present, all platform construction, team building and development planning are carried out around the development of independent intellectual property rights, bigger, stronger, and refined domestic CAR-T cell industry.
Li Hongjian, CEO of Purikin, said: "The core technology is not imitation, and it can't be bought."
Taking the route of independent intellectual property rights has become a leader in the CAR-T track, and Purikin has gained recognition from many parties. At ASCO, which just ended in June, Purikin made a poster presentation and received the attention of many foreign companies and doctors. Recently, the Chinese inspector officially released the "Quality Control Test for CAR-T Cell Therapy Products and the Considerations for Non-Clinical Research", and Preh Jin is one of the units that participated in the comments and suggestions.
Solid underlying technology platform for exporting diverse and stable products
Although there is no CAR-T therapy listed in China, the "Technical Guidelines for Research and Evaluation of Cellular Therapy Products (Trial)" document stipulates that cell therapy products should be evaluated according to drug research and development and registration, which means that CAR-T therapy companies must first The IND declares this.
Purikin has received the IND acceptance number for the targeted CD19 product of Kymriah using traditional antibodies. At present, the two CAR-T products that have been listed globally are targeted at CD19, and follow-up companies can reduce the risk of drug failure by starting from this target.
On the target of fewer people such as BCMA, Prekin also has a layout. Prehkin has a completely independent intellectual property right to target BCMA Nanobody CAR-T therapy, and it has a better effect in the treatment of multiple myeloma. Next, Purkin will target Nano-antibody CAR-T therapy targeting BCMA for MM. .
CAR-T therapy is currently very effective in the treatment of some leukemias and lymphomas, but the test progresses slowly in solid tumors. In addition, CAR-T therapy also has certain limitations and toxic side effects, which are in need of a reasonable solution. For example, when T cells kill other cells, they release a lot of proteins called cytokines. They can activate immune cells to fight against pathogens, but they can also cause a surprising immune response, a cytokine storm.
At present, Purkin has declared IND's "Chimeric Antigen Receptor T Cell Injection Targeting CD19", which has been modified in structure compared to the already-released Novartis Kymriah, and a safety switch has been added. After clinical research, the therapeutic effect of this product is comparable to that of Kymriah, but the safety is significantly improved, and there is no CRS (cytokine release syndrome) of grade 2 or higher.
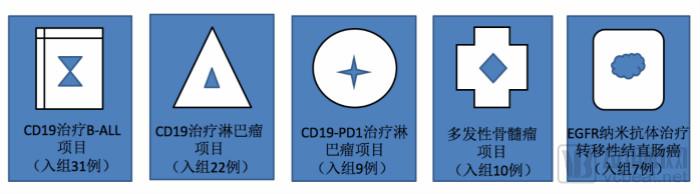
Puri Gold product line is rich
In terms of hard-to-break solid tumors, Prejin has been conducting in-depth research and development in conjunction with the latest international developments. Currently, it is working with Shenzhen Second People's Hospital to conduct a clinical study of EGFR-targeted Nanobody CAR-T in the treatment of metastatic colorectal cancer. The CAR-T conditionally expresses IL-12 and reverses the tumor immunosuppressive microenvironment. It is the only researched species in the world and is expected to achieve breakthrough in the treatment of solid tumors by CAR-T.
In addition to autonomous, mature and industrialized low-level platform technology, in addition to enriching product pipelines, Prejin can even provide various forms of CDMO services for other CAR-T R&D and production companies, including product development and production. Etc., build an open platform that provides a total solution for CAR-T and Nanobodies.
In addition to CAR-T, Nanobodies have great potential
The potential of CAR-T is huge, but Purikin's eggs are not all in one basket. Purikin's independent intellectual property nano-antibody platform can not only help develop better CAR-T therapy, but also has great market value. Independently become a medicine, or cooperate in the sale of Nanobodies.
It is reported that the global monoclonal antibody drug volume reached US$91.6 billion in 2015, with a growth rate of 7% in the year and is expected to reach US$112.2 billion in 2018. If Nanobodies can replace 50% of the market share, the global market capacity of Nanobodies will reach $56.1 billion. In terms of market competition, only Ablynx in Belgium is leading the global industry.
Nanobodies have the advantages of high affinity, high water solubility, high tolerance and stability compared to traditional antibodies. At the same time, Nano-antibodies have low immunogenicity and can be better "compatible" with human body. Nano-antibodies are weak in immunogenicity and biocompatible with humans because of their relatively small molecular mass and only one domain. Good sex.
In addition, Nanobodies have strong and fast tissue penetration, high expression, and easy removal. That is to say, it is possible to enter a dense tissue such as a solid tumor or the like. It is also possible to penetrate the blood-brain barrier and break through the bottleneck of the brain that cannot be used in the past. It is expected to become a new drug for the treatment of Alzheimer's disease.
Purikin's proprietary intellectual property nano-antibody platform, the important strength comes from the top founding team. Professor Li Shenghua, the co-founder of Prejin, is one of the first scientists in the world to work on nano-antibody research. He has more than 30 years of experience in research and development of biomedical products. Founder Zhang Jishuai, a postdoctoral fellow at the American Cancer Institute, has hosted the National Natural Science Foundation of China many times. After the team has accumulated a wealth of knowledge and research capabilities in antibodies and tumors, they want to realize the transformation of knowledge into clinical applications.
As the world's leading nano-antibody platform, Prejin also launched the "2018 First International New Antibody Drug Development Summit Forum", which will continue to be held annually.
Purikin CEO Li Hongjian said to the arterial network: "Because the accumulation of technology and professional resources has reached a certain level, Purikin believes that it has the obligation and responsibility to build a platform for communication and exchange, and can do something for the overall development of the industry. In addition to the use of Nano-antibodies for CAR-T cell drugs, it will also expand the field of nano-antibody drugs and diagnostics in a timely manner to create a nano-antibody technology industry chain."
We're Professional Supplier Extract Powder manufacturers and suppliers in China specialized in providing high-quality products at low price. We warmly welcome you to buy or wholesale bulk Supplier Extract Powder for sale here from our factory. For a free sample, contact us now.
Supplier Extract Powder,Supplier Extract ,Supplier Powder Manufacturer in China
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.bio-pharmacies.com