The French biomedical company Kalmar said on November 30 that the world's fifth permanent artificial heart transplant that had undergone surgery at the end of August this year had passed away. The medical analysis that has been completed does not show that the patient's death is related to the operation of the artificial heart.
At present, the French National Agency for Drug and Health Product Safety has requested Kalmar to stop all artificial heart transplants and not restart relevant clinical trials until the cause of death has been identified.
According to French media reports, the patient was undergoing an artificial heart transplant in a hospital near the southern French city of Nantes. He died in October after about a month and a half. This is the first patient to participate in the second phase clinical trial of the Kalmar Artificial Heart. Previously, four patients who participated in the Phase I clinical trial had died after a period of survival.
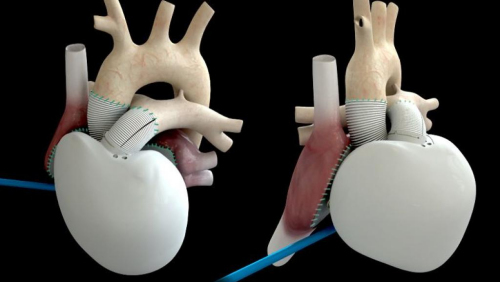
Since the end of 2013, Kalmar has implanted artificial heart in four patients with advanced heart failure in France, completing a phase I clinical trial to verify its safety, with the goal of surviving patients for 1 month after surgery. the above. In January of this year, as the company announced that the fourth transplant had died due to complications, the first phase of clinical trials officially ended, all four transplanted patients died, and the total survival time was 21 months.
According to Kalmar, the deaths of the first two patients in the four transplants were caused by slight infiltration of blood into the artificial heart-driven fluid, causing the artificial heart electronic drive to malfunction, while the artificial heart implanted in the latter two patients was always functioning properly.
Hydrophilic Introducer Sheath Kit
Hydrophilic Introducer Sheath,introducer sheath,Hydrophilic,arterial sheath introducer
Anesthesia Medical Co., Ltd. , https://www.medicaldiverse.com