New process for drug impurity identification - Q Exactive Focus combined with Compound Discoverer for pantoprazole impurity profile analysis
Key words
Drug impurity identification; Compound Discoverer; Q Exactive Focus; electrostatic field orbitrap high resolution mass spectrometry
1. Introduction
Any substance that affects the purity of a drug is collectively referred to as an impurity. Technical Requirements for Human Drug Registration International Coordination (ICH) defines impurities as any component present in a drug that is inconsistent with the drug. Impurities in the drug will reduce the efficacy, affect the stability of the drug, and some may even be harmful to human health or produce other toxic side effects. Therefore, the detection of related substances, control of purity to ensure the safety and effectiveness of medication, is very important to ensure the quality of the drug.
Mass spectrometry has been widely used for drug impurity identification due to its fast, high sensitivity and high specificity analysis capabilities. OrbitrapTM electrostatic field orbitrap high resolution mass spectrometry has high resolution and long-term stable high quality accuracy. High-quality first- and multi-stage high-resolution mass spectrometry data ensure the reliability of the identification results and are increasingly used in qualitative analysis.
In this paper, Thermo ScientificTM high performance liquid chromatography-quadrupole electrostatic field orbitrap Q ExactiveTM Focus high resolution mass spectrometry was used to collect the impurity data of pantoprazole, and the target compound was synthesized by high performance quadrupole. High-level attribute selection, HCD high-energy collision cell for secondary collision fragmentation, Orbitrap electrostatic field orbit trap for primary and secondary high-resolution mass spectrometry data. Combined with Thermo's next-generation smart small molecule compound analysis software, Compound DiscovererTM, the pantoprazole impurity analysis workflow was developed in a highly flexible and customizable way (Figure 1).
Figure 1. Impurity identification process based on Q Exactive Focus and Compound Discoverer
2. Experimental conditions
2.1 liquid chromatography conditions:
Instrument: Thermo ScientificTM UltiMateTM 3000 Ultra Performance Liquid Chromatograph
Column: Hypersil Gold C18 (100 x 2.1 mm, 1.9 μm)
Mobile phase: A is the aqueous phase: 0.1% formic acid water; B is the organic phase: acetonitrile
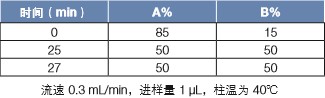
Gradient conditions:
2.2 Mass spectrometry conditions
Instrument: Thermo ScientificTM Q Exactive Focus Quadrupole Electrostatic Field Orbitrap High Resolution Tandem Mass Spectrometry. HESI ion source parameters: Spray Voltage +3.5 KV, 2.8 KV, fast positive and negative switching; Sheath Gas Pressure: 40arb; Aux Gas Pressure: 10arb; Capillary Temp: 300°C; Heater Temp: 350°C
Mass spectrometry scan parameters: full scan range 150–800 m/z, resolution 70,000 FWHM; secondary: Discovery data dependent auto-trigger; parent ion isolation window 0.4 amu, 4.0 amu; resolution 17,500, 70,000 FWHM.
2.3 sample preparation
Take appropriate amount of tolazole influencing factors, and add acetonitrile: water (50:50) to 0.2 mg/mL and then analyze the sample.
3 . result
3.1 Q Exactive Focus provides quality, comprehensive and efficient raw data
3.1.1 Fast positive and negative switching to enhance compound identification coverage
Q Exactive Focus has fast and efficient positive and negative switching capabilities. Taking the peak of 7.16 min in Figure 2 as an example, the [M+H]+ signal of 336.11500 m/z impurity is detected in positive ion mode, and the corresponding 334.10177 m/z [MH]- signal is detected in negative ion mode. In addition, another [M'-H]- signal of 320.08543 m/z was detected, indicating that the fast positive-negative switching acquisition mode can obtain comprehensive information of positive and negative ion impurities at the same time, and can be seen by the mass deviation of Figure 1. Whether in positive ion or negative ion mode, Q Exactive Focus maintains excellent mass accuracy and provides a strong guarantee for accurate identification.
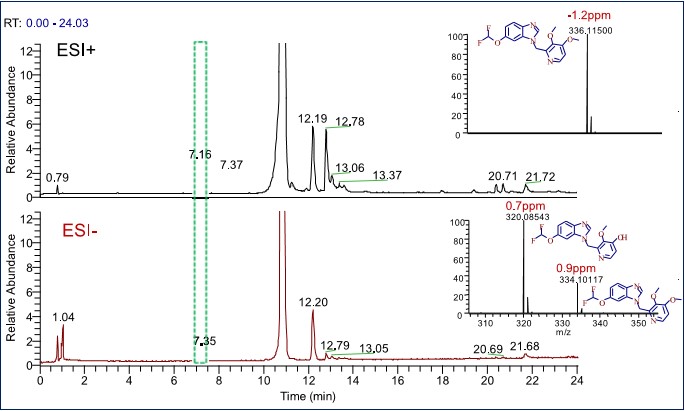
Figure 2. Fast positive and negative switching to detect impurities of different nature
3.1.2 More accurate quadrupole isolation of parent ions enhances the characteristics of the secondary spectrum
In the case of secondary fragmentation, it is necessary to use a quadrupole to screen out the parent ion, send it into the collision cell to collide and fragment, and obtain a two-stage high-resolution mass spectrum. Q Exactive Focus uses a high-performance hyperbolic quadrupole with as low as The mother ion selection ability of 0.4 amu, Figure 3 compares the mass spectrum of impurities of 367 m/z at different parent ion isolation widths, and there are many interferences of 122, 152, 184, 368 m/z plasma at 4.0 amu isolation width. When the isolation width is reduced to 0.4 amu, the interference is effectively removed, and from the viewpoint of the intensity of the secondary mass spectrometry, there is no significant difference between 0.4 amu and 4.0 amu. It shows that the high-performance quadrupole can improve the quadrupole resolution and more accurately select the parent ion without affecting the sensitivity, and obtain a more characteristic secondary mass spectrum to improve the accurate identification rate.
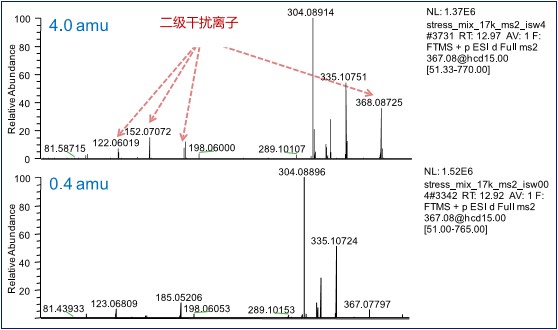
Figure 3. Comparison of secondary mass spectrometry data at different isolation widths
3.1.3 Obtaining characteristic isotope information at ultra-high resolution
When the resolution of the mass spectrum is high enough, the isotope peaks of the compound are further separated, taking Pantoprazole as an example (Figure 4), at a resolution of up to 70,000 FWHM at Q Exactive Focus, in the A2 isotope of the first-order mass spectrometer. 34S and 18O+13C2 can be separated. By filtering the 34S characteristic isotope signal, the pantoprazole-related substance signal containing S element can be selected from the total ion chromatogram, and the related substances can be identified more specifically.
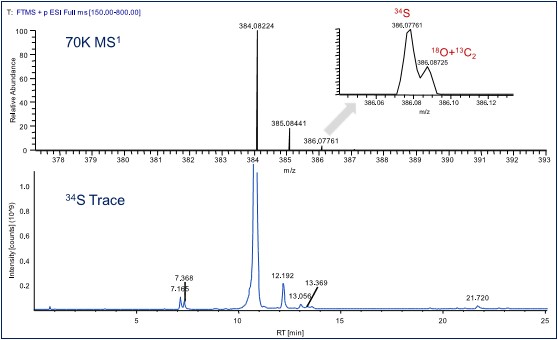
Figure 4. Resolution of the tosoptopazole 34S isotope peak separation and filtration at a resolution of 70,000 FWHM
3.2 Compound Discoverer data processing software to discover and identify targets and unknown impurities
3.2.1 Intelligent sample grouping compare different batch samples
Compound Discoverer can establish specific research programs for different projects. In the description of pantoprazole research, input the analysis conditions for the project; in the research factor, according to the nature of the sample, set three classification factors such as high temperature, high humidity and normal conditions. The imported samples are defined according to the classification factors, and the software can be intelligently grouped (Figure 5).
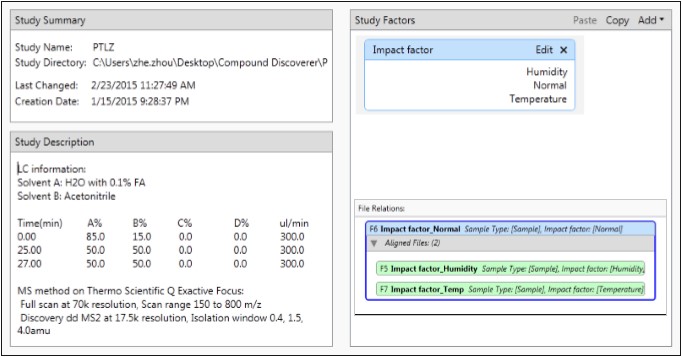
Figure 5. Compound Discoverer Pantoprazole Research Program
3.2.2 Flexible Customized Known and Unknown Impurity Data Processing Flow
In the Compound Discoverer workflow tree, you can flexibly edit the data processing flow according to your research needs. For the impurity analysis of pantoprazole, the known and unknown two main processes were used to extract and analyze the expected impurities and unknown impurities that may exist (Fig. 6).
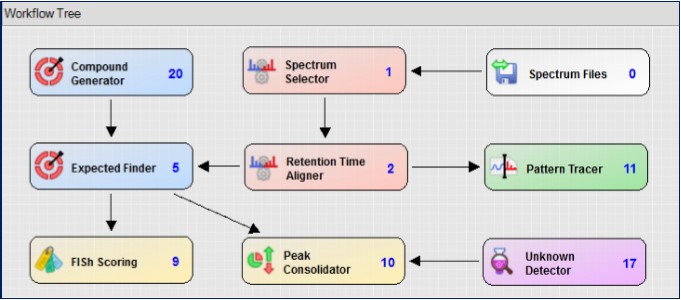
Figure 6. Impurity analysis workflow tree
3.2.3 Quick overview of impurity extraction analysis results
In the summary list of Compound Discoverer processing results, the expected and unknown impurity peak processing results can be viewed and screened intuitively and conveniently. From the result interface, the extracted ion current map of the impurity under different influencing factors can be visually compared, and then The retention time and response were compared; the mass accuracy of the primary mass spectrum was compared to the isotope and theoretical values ​​in the mass spectrum (Figure 7).
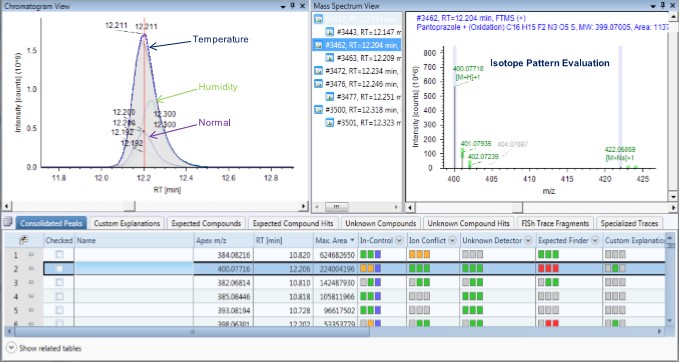
Figure 7. Data Processing Results Screen
3.2.4 Analysis and identification of unknown impurity fragments
After obtaining the primary and secondary mass spectral information of the parent drug and impurities, the software will call the Fragmentation Library to quickly assign the fragment structure of pantoprazole, which covers almost all published literature. The accuracy of fragmentation analysis is guaranteed. On the basis of the results of this study, the impurity-changing sites can be further speculated by software comparison of impurity and pantoprazole secondary mass spectrometry information (Fig. 8). The impurity was presumed to be pantoprazole sulfone by comparison of the common fragments such as 152 and 185 with the characteristic difference fragments of 200 and 216 (Fig. 9).
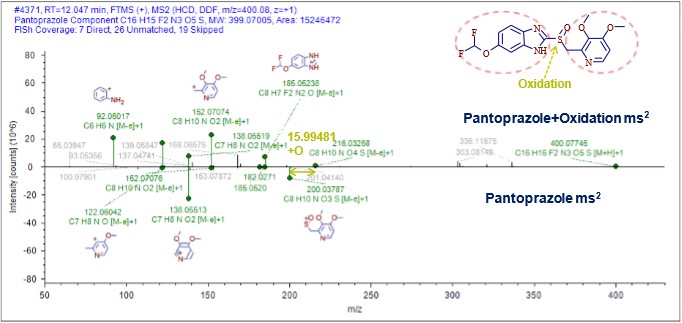
Figure 8. Secondary mass fragment fragmentation alignment results
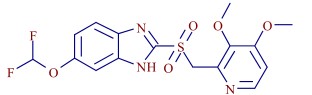
Figure 9. Impurity pantoprazole sulfone
3.3 æ³® torazole impurity analysis results
Using the above analytical procedure, a total of 15 pantoprazole impurities were detected, of which 9 were possible degradation impurities and 6 possible process impurities (Fig. 10).
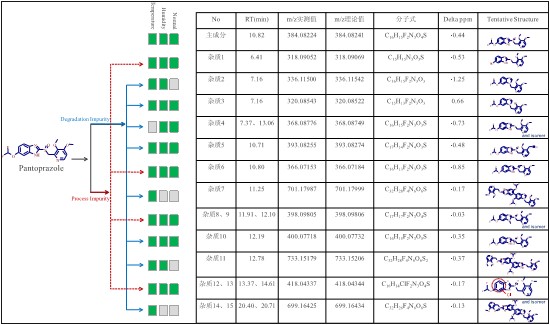
Figure 10. Summary of pantoprazole impurity classification and identification results
4. Summary
4.1 Q Exactive Focus mass spectrometer combines high-performance quadrupole with high-resolution electrostatic field orbitrap Orbitrap detection technology to achieve fast and positive switching with high quality accuracy and sensitivity for the most comprehensive impurity information. The high-performance quadrupole is able to accurately and quickly screen the parent ion to obtain a high-quality, pure secondary mass spectrum. Orbitrap technology has the ability to achieve high resolution and high precision (HR/AM). In this experiment, the paratolazole impurity has a mass deviation of < 1.5 ppm, which can accurately form the impurity element composition. The ultra-high resolution of 70,000 FWHM can distinguish the A2 isotope. The 34S and 18O+13C2 are more targeted for the identification of substances containing S.
4.2 Compound Discoverer is Thermo's new generation of intelligent small molecule compound identification software that can be used to build specific research programs for different projects. According to the preset classification factor, the samples under different influencing factors are conveniently grouped; a customized workflow tree is set according to the research needs, and the expected possible impurities and unknown impurities are extracted and analyzed; and in the result browsing Intuitively obtain sample comparison results under different conditions. By invoking the rich Fragmentation Library, the pantolazole fragment structure can be quickly speculated and assigned accurately. By comparing the impurity and pantoprazole secondary mass spectrometry information, further analysis can be performed. Predicting the impurity change sites, and finally obtaining a comprehensive impurity identification result.
We are manufacturer of Skin Whitening in China, if you want to buy Kojic Acid Dipalmitate Powder,Ethyl Ascorbyl Powder,Tranexamic Acid Powder please contact us.
Now we have 3 GMP standard workshop, Meanwhile, the factory is equipped with the researching and quality inspection centre, with strong technology research and development strength. We also have 3 salesdepartments over 30 people and sell our products all over the world.
Skin Whitening Powder,Hexapeptide-2 Peptide Powder,Phenylethyl Resorcinol Powder,Cyclotetrasiloxane Octamethyl Powder










