Rat astrocyte culture can be: (1) basic research for nervous system development, differentiation and regeneration; (2) mechanism of up-regulation of neuroprotective protein by cerebral ischemic preconditioning; (3) brain injury and brain pain Research; (4) Functional studies of new proteins.
experimental method
- Enzymatic digestion
- Trypsin digestion
- Trypsin digestion
| Principle of experimental method | After primary culture of rat astrocytes, they were identified by immunocytochemical staining of glial fibrillary acidic protein (GFAP). After hypoxia D-Hank solution and hypoxic culture tank, OGD was used to stain with trypan blue and lactate. Hydrogenase (LDH) leakage rate was used to measure the degree of cellular damage. |
|---|---|
| Experimental Materials | Wistar rat suckling rat |
| Reagents, kits | FBS DMEM trypsin EDTA alcohol |
| Instruments, consumables | Ophthalmic scissors dropper centrifuge Petri dish CO2 incubator |
| Experimental procedure | First, the experimental steps 1. Newborn 1-2 days Wistar rat suckling rats, disinfected by 75% alcohol, and killed by decapitation. 2. Remove the brain and place the cold D-Hanks' balanced salt solution to remove the meninges and large blood vessels. 3. Separate the cerebral cortex from both sides and cut into 1 mm 3 size. Add 0.25% trypsin and incubate in a 37 ° C air bath for 10 min. 4. Stop digestion with DMEM medium containing 10% FBS, centrifuge (1 000 rpm, 10 min), and remove the supernatant. 5. Resuspend the pellet in DMEM medium containing 10% FBS, filter through a 75 μm sieve, collect the filtrate, centrifuge again for 10 min, collect the pellet and resuspend in DMEM medium containing 10% FBS to adjust the cell density to 1.5×. 10 6 /ml, planted in 75 cm 2 flasks. 6. After removing fibroblasts by differential adhesion for 1 hour, transfer the cell suspension to a new 75 cm 2 flask and continue to culture. Change the solution once every two days. 7. After 7 days, place the flask in a horizontal shaker (260 rpm, 2 h, 37 °C), dilute the microglia, place in the incubator for 1 hour, and place it on the horizontal shaker for 18 h. 8. Change the oligodendrocyte by liquid exchange, wash it twice with fresh medium, digest with 0.25% trypsin and 0.02% EDTA 1:1 mixture, and pass it for later use. Second, morphological observation Cell morphology and growth were observed under an inverted phase contrast microscope. Third, the cell growth curve is drawn The cells after passage were planted in a 24-well culture plate at a density of 2×10 4 /ml, and the cells were changed once every 3 days, and 3 wells were counted every 24 hours for 7 consecutive days. Identification of astrocyte glial cells V. Results A method for the identification of glial fibrillary acidic protein (GFAP) immunohistochemistry. The star glial cells were removed, washed with PBS for 3 times, fixed in 4% paraformaldehyde for 1 hour at room temperature, washed three times with PBS for 5 minutes, 0.3% H 2 O 2 for 30 minutes to remove endogenous Oxide, PBS was washed 3 times for 5 minutes each time, and goat serum containing 0.5% Triton X-100 was blocked for 30 minutes, rabbit anti-GFAP antibody (1:75) was added dropwise, and the cells were washed 3 times at 4 °C for 18 hours. 5 minutes, add biotin-labeled goat anti-rabbit IgG, room temperature for 20 minutes, wash PBS three times for 5 minutes each time, add horseradish enzyme labeled streptavidin working solution, room temperature for 20 minutes, PBS wash 3 times, each After 5 minutes, DAB develops color. 1. Morphological observation: Under light microscopy, the passaged astrocytes have strong refractive index and irregular shape, mainly polygonal, with large and flat cells, abundant cytoplasm, round or oval nucleus, and partial to the cell body. On one side, there are 1-2 nucleoli, there are several crude and short-branched primary neurites, some of the cell processes become thinner and longer, and after 6-7 days, the cells fuse into a single layer, which is consistent with the growth characteristics of glial cells, as follows Figure. 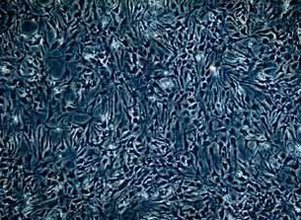 Star glio cell monolayer growth (×100)  Figure 1: Star glial cells form a fusion, single layer growth (×200) 2. Identification: cells stained with glial fibrillary acidic protein (GFAP) more than 98% are stained by cytoplasm, but no staining of nucleus, see the following figure, which proves that the cells obtained by the above method are astrocytes, purity High, can be used for experiments. 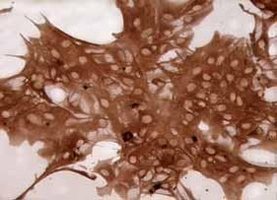 Figure 2: Immunohistochemical identification, GFAP staining 3. The cell growth curve is drawn as follows: 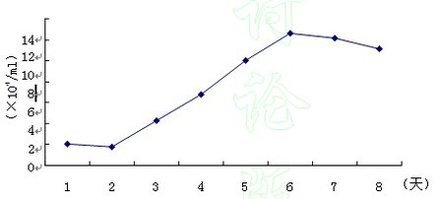 Figure 3: Cell growth curve |
| Experimental Materials | SD rat |
|---|---|
| Reagents, kits | Phenobarbital anatomical solution trypsin Dnase DM medium |
| Instruments, consumables | Centrifuge tube incubator surgical equipment |
| Experimental procedure | 1. Take one of 14.5d SD rats. Second, phenobarbital 0.5 ml intraperitoneal injection, placed on the operating table after anesthesia, alcohol cotton ball disinfection of the skin, open the uterus (about 12-14), into the anatomy, cut open the uterus, remove the embryo , placed in the anatomical solution, disconnected between the ear and eye, take the side of the head tissue, remove the meningeal tissue, take the original midbrain tissue, into the anatomical solution of the centrifuge tube, after all taken out, 1500 rpm 2 minute. 3. Aspirate the supernatant with a pipette and add a trypsin tube (1 ml/vial) to the tissue cell pellet at room temperature for 30 minutes, 1500 rpm, and centrifuge for 5 minutes (the anatomical solution can be added to terminate the trypsin before centrifugation). 4. Aspirate the supernatant, add a tube of Dnase (2 ml/vial) to the tissue cell pellet, react at 37 ° C for 10 minutes, centrifuge at 1500 rpm, centrifuge for 5 minutes, aspirate the supernatant, and add 2 ml of DM to the tissue cell pellet. The culture medium is blown, counted (counting formula is (N1+N2+N3+N4)/4*10 4 ), cultured in a culture flask at 3*10 5 / bottle, and marked (time, original or the first few Substitute generation, name, cell name, etc.), and cultured in an incubator. |
| Precautions | 1. The amount of anesthetic should be greater than the normal dosage. Expand  2. Care must be taken to remove the cleansing of the meninges, otherwise the purity of the cultured cells will be greatly affected. 3. Cell culture technology is different from molecular biology. Because of the slower centrifugation speed, the centrifuged tissue and cell adherence are not as tight as the nucleic acid. When the supernatant after centrifugation is taken out, it must be sucked with a pipette. It is forbidden to use the method of dumping. 4. To protect the embryo, the embryo should be handled in a tray in which ice cubes are placed, keeping it cool. 5. When taking the midbrain tissue, first use two tweezers to clamp the area and then cut it with a blade. 6. Trypsin is toxic to cells during cell centrifugation, so try to add a certain anatomical solution to dilute before centrifugation. 7. The cells are preferably centrifuged in a centrifuge to maintain cell viability. 8. Burn the pipette head with a fire before blowing the cells. When the redness is red, gradually retreat, making the tip of the pipette dull, reducing the damage to the cells during the blowing process. The experiment also found that there is a certain resistance when blowing. 9. When the mouse embryos are more, they can be digested in batches. Otherwise, the in vitro time is longer and the cell survival rate is lower. 10. Use Neurobasal medium + B27. 11. Adding EDTA during trypsin digestion can enhance digestion. 12. The key is the accuracy of the material and the state of the primary cells. |
| Experimental Materials | Rat embryo |
|---|---|
| Reagents, kits | L-15 medium collagenase Dnase D-MEM-BS FCS-DMEM cytarabine |
| Instruments, consumables | Centrifuge water bath culture flask incubator |
| Experimental procedure | I. Isolation of the neocortex of rat embryos (16-18 weeks), placed in L-15 (or Hank's balanced salt / DMEM solution, no Hepes) medium, remove meninges and blood vessels, hippocampus, caudate nucleus and other non-cortical tissues . 2. Cut it into 1 mm 3 tissue blocks with a scalpel. 3. Transfer 250 μl of collagenase stock solution (1.33%, dissolved in L-15) to 1 ml of L-15 (or D-MEM) using a 1 ml tip. The collagenase solution was diluted with 1-1.5 ml for every 2 brain tissue blocks. 4. Incubate for 30 min in a 37 ° C water bath. 5. Centrifuge for 5 min at 1000 rpm (approximately 230 g) or centrifuge for 1 min at 3 000 rpm (approximately 2 000 g). 6. Go to the supernatant. 7. Resuspend the cells in a solution containing EDTA-CMF and mix with trypsin at a ratio of 1:4 to 1:10. The ratio of the two can be appropriately adjusted depending on the observed cell digestion. 8. Add trypsin inhibitor and DNase solution and mix for 5 minutes. Centrifuge for 5 min at 9000 rpm (approximately 230 g) or centrifuge for 1 min at 3 000 rpm (approximately 2 000 g). Ten, go to the supernatant. Add 500 μl D-MEM-BS. Eleven, gently blow into a single cell suspension, start to blow 10 times with a 5 ml gun, and use a 18-gauge needle to blow when needed. (Note: Do not create air bubbles.) 200 mesh / 300 mesh nylon mesh filter. 12. Transfer the cells to a culture flask containing 10% FCS-DMEM, culture a culture flask with a density of 2× 10 6 /25 cm 2 , and a culture flask of 4-6 x 10 6 /75 cm 3 . Incubate at 37.2 ° C, 37.5%. Thirteen, change the liquid on the second day, and then change the liquid every three days. Fourteen, 7-10 days, the cells fuse. 15. After the cells are fused, the caps are sealed with a sealing film and cultured on a trajectory shaker. The rotation speed is preferably such that no bubbles are generated. Shake overnight at 37 °C. The cells are protected from light. 16. Remove the supernatant and add fresh 10% FCS-DMEM. 17. Add 200 μl of 1 mM cytarabine/10 ml medium. Incubation was carried out for 48 hours at 37.2 ° C, 37.5% to eliminate dividing cells. 18. Replace with fresh 10% FCS-DMEM and incubate for 24 hours. Nineteen, repeat steps 17-18 to eliminate all dividing cells |
Ginger Tea Granules,Ginger Granules ,Ginger Minced By Air Dried,Dried Ginger
Shanghai Sinospices Sourcing Ltd , https://www.garlicall.com