
Driven by the interests, in recent years, there have been frequent reports that worms and worms have been spliced, excessive heavy metals have been exceeded, and falsifications have occurred. In the aspect of Cordyceps supervision, the food and drug supervision department usually arranges random inspections, and each year special actions are carried out against expensive fine medicinal materials, but due to the special nature of Cordyceps (triple attributes of traditional Chinese medicine, health food raw materials, and traditional tonics), it is currently regulated by Cordyceps. There is still a certain vacuum in the area.
Triple attribute
Cordyceps has a long history of clinical application. In the traditional Chinese medicine theory, Cordyceps has the effect of nourishing yin and yang. According to report, as early as 2001, the Ministry of Health had explicitly restricted the use of secondary protected species such as Cordyceps sinensis as a raw material for health foods. In 2005, the State Food and Drug Administration (SFDA) took over the supervision of health foods and further clarified that if Cordyceps sinensis is used as a raw material for health food, it should be replaced with artificially propagated mycelium. In August 2012, SFDA released the "Cocktail Work Program for Cordyceps sinensis for Health Food", which allowed Cordyceps sinensis to be directly used as a raw material for health food for the first time.
In addition, Cordyceps, ginseng, and velvet are all traditional tonics. The intersection of triple identities makes Cordyceps supervision difficult. Zou Xiaorong, deputy director of the Huai'an Food and Drug Administration in Jiangsu Province, told reporters: “The regulatory issue of Cordyceps is more complicated. There are no clear rules on how to define medicines and non-drugs. There are two main approaches in law enforcement practice. Point to see if the packaging, labels, and instructions indicate functional indications and promote efficacy; second, see whether formulas, otherwise it will be treated as a traditional supplement.â€
Cordyceps Tablets Herbal Pieces
It is understood that Cordyceps's existing decoction standards include the standards in the “Chinese Pharmacopoeia†and the regulations of the provinces. In the 2010 edition of the Pharmacopoeia, Cordyceps sinensis standard: "Cleaning Cordyceps sinensis, taking the original herbs to remove impurities, washing, low-temperature drying, low-temperature sterilization. Cordyceps sinensis powder to take the original herbs to remove impurities, wash, low temperature drying, low temperature Sterilize and smash it."
Recently, due to the appearance of Cordyceps sinensis films, the “Code for Processing Chinese Herbal Cordyceps Sinensis in Qinghai Province†published on December 31, 2010 in Qinghai Province has been controversial. After consulting the website of the Food and Drug Administration of Qinghai Province, the reporter was informed that the following are included in the concoction of Cordyceps Pieces: "Cordyceps sinensis pure powder tablets to take the original herbs to remove impurities, washed, low temperature drying, low temperature sterilization, smash, pure powder Press into a piece."
Some work in the inspection line of food and drug regulatory staff put forward, "Cordyceps pure powder tablet is a piece of fresh or preparation is the focus of controversy." The reporter learned that the cordyceps pure powder tablets in Qinghai Province have not been supplemented with tablets such as dextrin and starch and cannot be considered as new formulations. If it is a new preparation, SFDA approval is required, and different processing specifications can be formulated by the provincial food and drug regulatory authorities.
An inspector in the QQ Group of the National Food and Drug Inspection stated: “There are two licenses for the production of pharmaceuticals, one is production license (enterprise production qualification), and the other is product license (specific drug registration certificate, ie, Zhuozi Quasi). Chinese herbal medicines are not compulsory licenses, and the determination of the scope of application of the recipes for Chinese herbal medicines is based on the specifications for Chinese herbal medicine prepared and promulgated by the drug regulatory agencies of provinces, autonomous regions, and municipalities directly under the Central Government, which are only applicable to the production, sales, and inspection of Chinese herbal medicines in their jurisdictions. Etc.' to perform."
Call for qualitative supervision
The regulation of Cordyceps is divided into two aspects: quality supervision and licensing supervision. A person inspecting the front line put forward: “Quality supervision is mainly for the identification of Pseudo-Chong Cordyceps. There are many fake P. edodes on the market, and the main counterfeit species are the sub-scented Cordyceps sinensis. The distinguishing point is that the genuine ring of insects is obvious and the gastropod is obvious. However, the sub-aromatic rods have no obvious rings and gastropods, and there are black pores in the parasites. Cordyceps sold according to pharmaceuticals has no difficulty in supervision and can supervise sampling. The main problem at present is the regulation of business qualifications, that is, because there are no other non-drug standards. Does Cordyceps have the property of only one drug in the management category? Apart from drugs, can it be sold as other products? This series of questions puzzles the auditing frontline staff."
Cordyceps market chaos, regulate the market and strengthen supervision is equally important and urgent. "The drug regulatory department can only supervise products that are managed by drugs. If it is not possible to determine the unique category attribute of Cordyceps by drug management, companies such as health care products stores can treat them as non-pharmaceutical products, and the drug administration department will have no jurisdiction." He pointed out.
Industrial Laser Distance Sensor
Industrial Laser Distance Sensor, we also call it secondary development laser distance module, which support TTL level and CMOS. The laser range sensor can be widely used in professional surveying, mapping, construction, robots, hunting arrows, industrial monitoring and automated measurement applications in electricity, transportation, etc. Our laser distance module supports data communication with RS232, USB with a simple adapter. The results of laser distance sensor can be evaluated with Arduino. We are always looking ahead, hoping we can make every measurement simple in life!
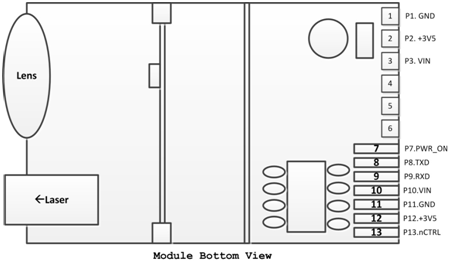
Parameters of M703A:
|
Accuracy |
±1 mm (0.04 inch) |
|
Measuring Unit |
meter/inch/feet |
|
Measuring Range (without Reflection) |
0.03-150m |
|
Measuring Time |
0.1~3 seconds |
|
Laser Class |
Class II |
|
Laser Type |
635nm, <1mW |
|
Size |
72*40*18mm (±1 mm) |
|
Weight |
About 21g |
|
Voltage |
DC2.0~3.3V |
|
Electrical Level |
TTL/CMOS |
|
Frequency |
10Hz |
|
Operating Temperature |
0-40 ℃ (32-104 ℉ ) |
|
Storage Temperature |
-25~60 ℃ (-13~140 ℉) |
Laser Distance RS232,Arduino Distance Module,Laser Module RS232
Chengdu JRT Meter Technology Co., Ltd , https://www.rangingsensor.com